
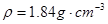 £© 60mL³ä·Ö·“Ó¦£¬ŠæČ«²æČܽā£¬¶ŌÓŚÖʵƵÄĘųĢ壬ӊĶ¬Ń§ČĻĪŖæÉÄÜ»ģÓŠŌÓÖŹ”£
£© 60mL³ä·Ö·“Ó¦£¬ŠæČ«²æČܽā£¬¶ŌÓŚÖʵƵÄĘųĢ壬ӊĶ¬Ń§ČĻĪŖæÉÄÜ»ģÓŠŌÓÖŹ”£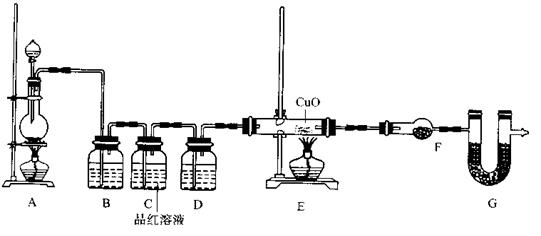
 2Fe3+ £Ø2·Ö£©
2Fe3+ £Ø2·Ö£©  2Fe3+
2Fe3+ 


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗ¼ĘĖćĢā
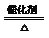 2SO3
2SO3²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ÅØĮņĖįÓėŃĒĮņĖįÄĘ·“Ó¦ÖĘČ”¶žŃõ»ÆĮņ£¬ĢåĻÖÅØĮņĖį¾ßÓŠĒæŃõ»ÆŠŌ |
| B£®ļ§ŃĪŹÜČČŅ×·Ö½ā£¬¾łÓŠNH3²śÉś |
| C£®ÅØĻõĖį±£“ęŌŚ×ŲÉ«µÄĻøæŚĘæÖŠ |
| D£®»Æ¹¤³§æÉĶعż¼ÓøßŃĢ“ŃÅÅ·Å·ĻĘų£¬·ĄÖ¹ŠĪ³ÉĖįÓź |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®µ„ÖŹ¹čŹĒ½«Ģ«ŃōÄÜ×Ŗ»ÆĪŖµēÄܵij£ÓĆ²ÄĮĻ |
| B£®¹čĖįÄʵÄĖ×ĆūĪŖ²£Į§£¬æÉÓĆÓŚÖʱø¹č½ŗŗĶľ²Ä·Ą»š¼Į |
| C£®ÓĆÅØH2SO4æĢŹ“¹¤ŅÕ²£Į§ÉĻµÄĪĘŹĪ |
| D£®SO2¾ßÓŠŃõ»ÆŠŌ£¬æÉÓĆĄ“ĘÆ°×Ö½½¬ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
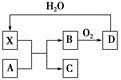
| A£®XŹ¹ÕįĢĒ±äŗŚµÄĻÖĻóÖ÷ŅŖĢåĻÖĮĖXµÄĶŃĖ®ŠŌ |
| B£®ČōAĪŖĢś£¬Ōņ×ćĮæAÓėXŌŚŹŅĪĀĻĀ¼“æÉĶźČ«·“Ó¦ |
| C£®ČōAĪŖĢ¼µ„ÖŹ£¬Ōņ½«CĶØČėÉŁĮæµÄ³ĪĒåŹÆ»ŅĖ®ÖŠ£¬Ņ»¶ØæÉŅŌ¹Ū²ģµ½°×É«³Įµķ²śÉś |
| D£®¹¤ŅµÉĻ£¬B×Ŗ»ÆĪŖDµÄ·“Ó¦Ģõ¼žĪŖøßĪĀ”¢øßŃ¹”¢Ź¹ÓĆ“ß»Æ¼Į |
²éæ““š°øŗĶ½āĪö>>
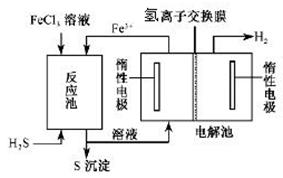
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
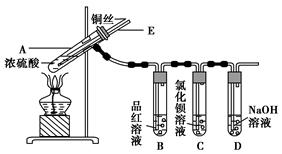
| µĪ¼ÓµÄČÜŅŗ | ĀČĖ® | °±Ė® |
| ³ĮµķµÄ»ÆѧŹ½ | | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
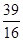 a£„ c£®
a£„ c£®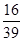 (1-a£„) D£®ĪŽ·Ø¼ĘĖć
(1-a£„) D£®ĪŽ·Ø¼ĘĖć²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com