
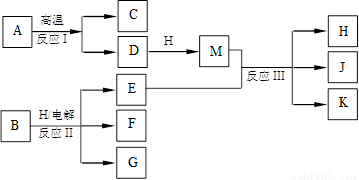
��ͼ��ʾ��ӦI����ӦII�ͷ�ӦIII���ǹ�ҵ�����г����ķ�Ӧ������A��BΪ�����C����������֮һ��D��K���������������H������ΪҺ̬�����J��һ�־���Ư�����õ��Σ���ӦIII��E��G��Ӧ��ԭ����ͬ��

��1��C��J��ˮ��Һ��Ӧ�����ɵĺ�����ĵ���ʽ������������������ ��
��2��E��G��Ӧ�����ӷ���ʽ������������������������������������������ ��
��3��J���ú��㲻�Ӵ�ˮ������������Ҳ�ֽ�����K�����ų����壬�÷�Ӧ�Ļ�ѧ����ʽ�������������������������������� ��
��4����ҵ�ϲⶨ��ӦIII��Ʒ����Ч�ɷ�J�ĺ������Ƚ�һ�����IJ�Ʒ����Һ���������KI��Һ��ϡ�����У�ʹ֮��Ӧ����I2��Ȼ����Na2S2O3����Һ�ζ�I2������������
����Na2S2O3����Һ�ζ�I2ʱѡ�õ�ָʾ�������������������� ��
������I2�ķ�Ӧ�����ӷ���ʽ������������������������������ ��
��5����֪��2Fe2+ + Br2��2Fe3+ +2Br��������0.1 mol Eͨ��100 mL FeBr2��Һ�У���Һ��������֮һ��Br����������Br2����˷�Ӧ���ӷ���ʽ���������������������������������� ��ԭFeBr2��Һ�����ʵ���Ũ��Ϊ������ ������ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��
�� 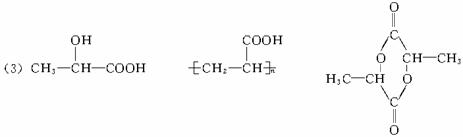
�ڽ�A������ͼ��ʾ�ķ�Ӧ������C��C����ͬϵ�C���Ǽ��ᣨHCOOH��
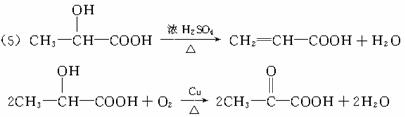
������������⣺
��1��C��D������____________�������ţ���ͬ��
��ͬϵ���ͬ���칹��
��ͬ�����ʢ�ͬ��������
��2��D���E�ֱ�������һ����D��____________��E____________��
�ٶ�Ԫ�� ��ȩ �۲��������� �ܶ�Ԫ���� ����
��3��д���ṹ��ʽ��
A___________________,F_______________��H_____________________��
��4��д����Ӧ���ͣ���ӦI____________����Ӧ��____________��
��5��д������ת���Ļ�ѧ����ʽ
A��E______________________________________________________________��
A��B______________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣���ͼ��ʾ��ӦI����ӦII�ͷ�ӦIII���ǹ�ҵ�����г����ķ�Ӧ������A��BΪ�����C����������֮һ��D��K���������������H������ΪҺ̬�����J��һ�־���Ư�����õ��Σ���ӦIII��E��G��Ӧ��ԭ����ͬ��
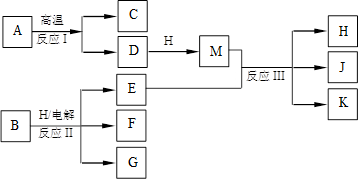
��1��C��J��ˮ��Һ��Ӧ�����ɵĺ�����ĵ���ʽ�� ��
��2��E��G��Ӧ�����ӷ���ʽ�� ��
��3��J���ú��㲻�Ӵ�ˮ������������Ҳ�ֽ�����K�����ų����壬�÷�Ӧ�Ļ�ѧ����ʽ�� ��
��4����ҵ�ϲⶨ��ӦIII��Ʒ����Ч�ɷ�J�ĺ������Ƚ�һ�����IJ�Ʒ����Һ���������KI��Һ��ϡ�����У�ʹ֮��Ӧ����I2��Ȼ����Na2S2O3����Һ�ζ�I2������������
����Na2S2O3����Һ�ζ�I2ʱѡ�õ�ָʾ���� ��
������I2�ķ�Ӧ�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ��������У������ѧ������������ѧ�Ծ� ���ͣ������
��10�֣���ͼ��ʾ��ӦI����ӦII�ͷ�ӦIII���ǹ�ҵ�����г����ķ�Ӧ������A��BΪ�����C����������֮һ��D��K���������������H������ΪҺ̬�����J��һ�־���Ư�����õ��Σ���ӦIII��E��G��Ӧ��ԭ����ͬ��
��1��C��J��ˮ��Һ��Ӧ�����ɵĺ�����ĵ���ʽ�� ��
��2�� E��G��Ӧ�����ӷ���ʽ�� ��
E��G��Ӧ�����ӷ���ʽ�� ��
��3��J���ú��㲻�Ӵ�ˮ������������Ҳ�ֽ�����K�����ų����壬�÷�Ӧ�Ļ�ѧ����ʽ�� ��
��4����ҵ�ϲⶨ��ӦIII��Ʒ����Ч�ɷ�J�ĺ������Ƚ�һ�����IJ�Ʒ����Һ���������KI��Һ��ϡ�����У�ʹ֮��Ӧ����I2��Ȼ����Na2S2O3����Һ�ζ�I2������������
����Na2S2O3����Һ�ζ�I2ʱѡ�õ�ָʾ���� ��
������I2�ķ�Ӧ�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ��������У������ѧ������������ѧ�Ծ� ���ͣ������
��10�֣���ͼ��ʾ��ӦI����ӦII�ͷ�ӦIII���ǹ�ҵ�����г����ķ�Ӧ������A��BΪ�����C����������֮һ��D��K���������������H������ΪҺ̬�����J��һ�־���Ư�����õ��Σ���ӦIII��E��G��Ӧ��ԭ����ͬ��
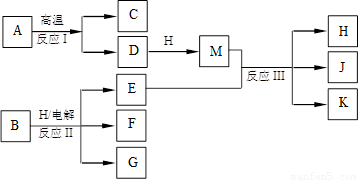
��1��C��J��ˮ��Һ��Ӧ�����ɵĺ�����ĵ���ʽ�� ��
��2��E��G��Ӧ�����ӷ���ʽ�� ��
��3��J���ú��㲻�Ӵ�ˮ������������Ҳ�ֽ�����K�����ų����壬�÷�Ӧ�Ļ�ѧ����ʽ�� ��
��4����ҵ�ϲⶨ��ӦIII��Ʒ����Ч�ɷ�J�ĺ������Ƚ�һ�����IJ�Ʒ����Һ���������KI��Һ��ϡ�����У�ʹ֮��Ӧ����I2��Ȼ����Na2S2O3����Һ�ζ�I2������������
����Na2S2O3����Һ�ζ�I2ʱѡ�õ�ָʾ���� ��
������I2�ķ�Ӧ�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ������ѧ�ڵ�����ģ�⿼�Ի�ѧ�Ծ� ���ͣ������
��10�֣���ͼ��ʾ��ӦI����ӦII�ͷ�ӦIII���ǹ�ҵ�����г����ķ�Ӧ������A��BΪ�����C����������֮һ��D��K���������������H������ΪҺ̬�����J��һ�־���Ư�����õ��Σ���ӦIII��E��G��Ӧ��ԭ����ͬ��
��1��C��J��ˮ��Һ��Ӧ�����ɵĺ�����ĵ���ʽ�� ��
��2��E��G��Ӧ�����ӷ���ʽ�� ��[��Դ:]
��3��J���ú��㲻�Ӵ�ˮ������������Ҳ�ֽ�����K�����ų����壬�÷�Ӧ�Ļ�ѧ����ʽ�� ��
��4����ҵ�ϲⶨ��ӦIII��Ʒ����Ч�ɷ�J�ĺ������Ƚ�һ�����IJ�Ʒ����Һ���������KI��Һ��ϡ�����У�ʹ֮��Ӧ����I2��Ȼ����Na2S2O3����Һ�ζ�I2������������
����Na2S2O3����Һ�ζ�I2ʱѡ�õ�ָʾ���� ��
������I2�ķ�Ӧ�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com