��1��298K��100kPaʱ��C��s��ʯī��+O
2��g���TCO
2��g����H
1=-393.5kJ?mol
-1 2H
2��g��+O
2��g��=2H
2O��l����H
2=-571.6kJ?mol
-1 2C
2H
2��g��+5O
2��g��=4CO
2��g��+2H
2O��l����H
3=-2599kJ?mol
-1����д��298Kʱ��C��s��ʯī����H
2��g������1molC
2H
2��g�����Ȼ�ѧ����ʽ
C��s��ʯī��+H2��g��=C2H2��g����H=226.7kJ?mol-1
C��s��ʯī��+H2��g��=C2H2��g����H=226.7kJ?mol-1
��
��2��ˮ������֮Դ��Ҳ�ǻ�ѧ��Ӧ�е����ǣ��Իش��������⣺A��B��C����ѧ��ѧ������������ɫ���ʣ�����ɵ�Ԫ�ؾ���������Ԫ�أ������Ǿ�����ˮ����������ԭ��Ӧ����ˮ�Ȳ���������Ҳ���ǻ�ԭ������д��A��B��C��ˮ��Ӧ�Ļ�ѧ����ʽ��
3NO2+H2O=2HNO3+NO��Cl2+H2O=HCl+HClO��2Na2O2+2H2O=4NaOH+O2��
3NO2+H2O=2HNO3+NO��Cl2+H2O=HCl+HClO��2Na2O2+2H2O=4NaOH+O2��
��
��3��д��������ˮ��Һ��ˮ������ӷ���ʽ
S
2-+H
2O
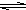
HS
-+OH
-S
2-+H
2O
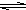
HS
-+OH
-������������Һʱ��Ϊ�˷�ֹ����ˮ�⣬���Լ���������
NaOH
NaOH
��
��4������������ˮ������Ϊ
Al3+ˮ������Ľ�״��Al��OH��3���������ԣ���������ˮ�е�����
Al3+ˮ������Ľ�״��Al��OH��3���������ԣ���������ˮ�е�����
���йص����ӷ���ʽΪ
Al
3++3H
2O
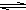
Al��OH��
3+3H
+����ɫ����Ͱ�ɫ��״��������
Al
3++3H
2O
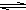
Al��OH��
3+3H
+����ɫ����Ͱ�ɫ��״��������
����������ˮ��Һ�м��뱥�͵�С�մ���Һ����۲쵽��������
����ɫ����Ͱ�ɫ��״��������
����ɫ����Ͱ�ɫ��״��������
���йص����ӷ���ʽ
3HCO3-+Al3+=Al��OH��3��+CO2��
3HCO3-+Al3+=Al��OH��3��+CO2��
��
��5��������Щ��ʵ��˵������������
�ڢܢݢ�
�ڢܢݢ�
�ٴ����ʴ�·���
��0.1mol/L��CH
3COONa��Һ��PHԼΪ9��
�۽����к͵ζ�ʱ������������ʵ���Ũ�ȵ�H
2SO
4��Һ�ȵ���������ʵ���Ũ�ȵ�CH
3COOH��Һ���ĵ�NaOH��Һ�ࣻ
��0.1mol/L��CH
3COOH��ҺPHԼΪ2.9��
����ͬ�����PH������4�������CH
3COOH��Һ����ͬһ���ʵ���Ũ�ȵ�NaOH��Һ�кͣ�CH
3COOH��Һ���ĵ�NaOH��Һ�ࣻ
��þ����һ����ϡ���ᷴӦ����������м������������ƿ��Խ��ͷ�Ӧ���ʵ����ı���������������




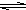 HS-+OH-
HS-+OH-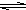 HS-+OH-
HS-+OH-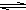 Al��OH��3+3H+����ɫ����Ͱ�ɫ��״��������
Al��OH��3+3H+����ɫ����Ͱ�ɫ��״��������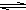 Al��OH��3+3H+����ɫ����Ͱ�ɫ��״��������
Al��OH��3+3H+����ɫ����Ͱ�ɫ��״��������