
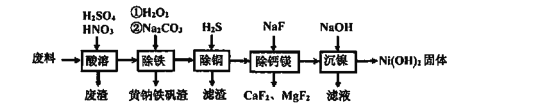
����Ŀ������(Ni)��������Լ20%���ϣ���Ҫ�ɷ��������Ͻ𣬻�����ͭ���ơ�þ�����������ɸ÷����Ʊ����Ƚϸߵ����������������������£�
�ش��������⣺
(1)�Ͻ��е���������ϡ���ᣬ��������ʱ���˼���ϡ���ᣬ��Ҫ�߽����������ϡ���ᣬ��Ӧ��N2���ɡ�д���������ܽ�Ļ�ѧ����ʽ______________________
(2)��������ʱH2O2��������________________��Ϊ��֤�����ӵ�H2O2��������Ӧѡ����Լ���_______________ (����ţ��������軯��K3[Fe(CN)6]�����������軯��KSCN��)��Һ����������[NaxFey(SO4)m(OH)n]���г��������������ʿ졢�����˵��ص㣬��x��y��m��n=1��____��2��6
(3)����ͭ��ʱ����Ӧ�����ӷ���ʽΪ________________
(4)��֪Ksp(MgF2)=7.35��10-11��Ksp(CaF2)=1.05��10-10�����������NaF������þ����������Һ��![]() ________________(����1λС��)����֪���ӹ������մ������н��У�NaF��ʵ���������˹����ԭ����__________
________________(����1λС��)����֪���ӹ������մ������н��У�NaF��ʵ���������˹����ԭ����__________
(5)100kg���Ͼ����������Ƶ�Ni(OH)2���������Ϊ31kg������������Ϊ______________(����1λС��)
(6)�������ѳ�Ϊ��϶�����������Ҫ������ͣ��乤��ԭ�����£�M+Ni(OH)2![]() MH+NiOOH(ʽ��MΪ����Ͻ�)��д����س������������ĵ缫��Ӧʽ___________
MH+NiOOH(ʽ��MΪ����Ͻ�)��д����س������������ĵ缫��Ӧʽ___________
���𰸡�5Ni+5H2SO4+2HNO3=5NiSO4+N2��+6H2O ��Fe2+��������Fe3+ �� 3 H2S+Cu2+=CuS��+2H+ 0.7 ������F-�����������»ḯʴ�մ����� 98.3% Ni(OH)2+OH-��e-=NiOOH+H2O
��������
������������Լ20%�ķ��ϣ���Ҫ�ɷ��������Ͻ𣬻�����ͭ���ơ�þ��������������������������ܣ��Ͻ��е���������ϡ���ᣬ��������ʱ���˼���ϡ���ᣬ��Ҫ�߽����������ϡ���ᣬ�ܽ�Ni��Ӧ��N2���ɣ����˳�ȥ��������Һ�м����������������������Ϊ�����ӣ�����̼���Ƶ�����ҺpH��ȥ�����ӣ����˵õ���������Һ����Һ�м���H2S����ͭ���ӣ����˵õ���Һ�м���NaF������ȥþ���Ӻ����ӣ����˵õ���Һ����Ҫ�������ӣ���������������Һ�������������������������塣
��1���������ᷴӦ���������ӡ�������ˮ�����ԭ���غ㡢�����غ���ƽ��д��ѧ����ʽ��
��2����������������������Ϊ�����ӣ����ڳ�ȥ������Na2CO3��ȥFe3+�������������������ӣ��������軯�غ��������ӽ��������ɫ��Һ�������������Ƿ���ȫ�������������Ϣ����������[NaxFey��SO4��m��OH��n]Ԫ�ػ��ϼ۴�����Ϊ0��
��3�������ͭ���ӷ�Ӧ���������������ͭ������
��4�������ܶȻ�������Һ��Mg2+��Ca2+��Ũ��֮�ȣ�NaF���������˹�������Ϊ�����������ƻ���������Һ�����ɷ����⣬�մ������еĶ��������ͷ����ⷴӦ��
��5�����Ϻ�����������Լ20%��100kg���Ͼ����������Ƶ�Ni��OH��2���������Ϊ31kg��������Ԫ���غ������յõ�����ԭ��������������������ʣ�
��6��������Ni��OH��2ʧ��������NiOOH��
������������Լ20%�ķ��ϣ���Ҫ�ɷ��������Ͻ𣬻�����ͭ���ơ�þ��������������������������ܣ��Ͻ��е���������ϡ���ᣬ��������ʱ���˼���ϡ���ᣬ��Ҫ�߽����������ϡ���ᣬ�ܽ�Ni��Ӧ��N2���ɣ����˳�ȥ��������Һ�м����������������������Ϊ�����ӣ�����̼���Ƶ�����ҺpH��ȥ�����ӣ����˵õ���������Һ����Һ�м���H2S����ͭ���ӣ����˵õ���Һ�м���NaF������ȥþ���Ӻ����ӣ����˵õ���Һ����Ҫ�������ӣ���������������Һ�������������������������塣
��1���������ᷴӦ���������ӡ�������ˮ�����ԭ���غ㡢�����غ���ƽ��д��ѧ����ʽΪ��5Ni+5H2SO4+2HNO3=5NiSO4+N2��+6H2O��
�ʴ�Ϊ��5Ni+5H2SO4+2HNO3=5NiSO4+N2��+6H2O��
��2����������ʱH2O2�������ǣ���������������������Ϊ�����ӣ����ڳ�ȥ��Ϊ��֤�����ӵ�H2O2��������Ӧѡ����Լ����������軯�غ��������ӽ��������ɫ��Һ�������������Ƿ���ȫ�������ټ���Na2CO3ʹFe3+���ɻ�����������ȥ����������[NaxFey��SO4��m��OH��n]����Ԫ�ػ��ϼ�Ϊ+3�ۣ�Ԫ�ػ��ϼ۴�����Ϊ0��x+3y-2m-n=0���õ�x+3y=2m+n��x��y��m��n=1��p��2��6����p=3��
�ʴ�Ϊ����������������Ϊ�����ӣ��٣�3��
��3�������ͭ���ӷ�Ӧ���������������ͭ��������Ӧ�����ӷ���ʽΪ��H2S+Cu2+=CuS��+2H+��
�ʴ�Ϊ��H2S+Cu2+=CuS��+2H+��
��4��������Һ��![]() =
=![]() =
=![]() =
=![]() =0.7��NaF���������˹�������Ϊ�����������ƻ���������Һ�����ɷ����⣬�մ������еĶ��������ͷ����ⷴӦ�����������ḯʴ�մ�������
=0.7��NaF���������˹�������Ϊ�����������ƻ���������Һ�����ɷ����⣬�մ������еĶ��������ͷ����ⷴӦ�����������ḯʴ�մ�������
�ʴ�Ϊ��0.7��������F-�������������ḯʴ�մ�������
��5�����Ϻ�����������Լ20%��100kg���Ͼ����������Ƶ�Ni��OH��2���������Ϊ31kg����������Ԫ������=100kg��20%=20kg����Ӧ������Ԫ������=31��93��59kg�����������ʵļ���ʽ=(31��93��59)kg��20kg��100%=98.3%��
�ʴ�Ϊ��98.3%��
��6�������س��ʱ�ķ�ӦΪM+Ni��OH��2=MH+NiOOH���������������ĵ缫��Ӧʽ��Ni(OH)2+OH--e-=NiOOH+H2O���ʴ�Ϊ��Ni(OH)2+OH--e-=NiOOH+H2O��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ��������������Դ�������ֲ��ϵ�����ʾ��ͼ���¡�����˵������ȷ����(����)

A. ���������Ʊ��ϸߴ���Al�Ĺ����г��õ�NaOH��Һ��CO2���塢����ʯ
B. ʯ��ʯ�����ʯӢ�������������Σ����������ᷴӦ
C. ���ƴֹ�ʱ���������������뱻��ԭ�����ʵ����ʵ���֮��Ϊ2��1
D. ��ͭ��(CuFeS2)��O2��Ӧ������Cu2S��FeO���ǻ�ԭ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ṩ��п����ͭƬ��500 mL 0.4 mol��L��1��H2SO4��Һ�����ߡ�1000 mL��Ͳ��������ͼװ�����ⶨп��ϡ���ᷴӦʱ��ij��ʱ����ͨ�����ߵĵ��ӵ����ʵ�����

��1����ͼ��ʾ��װ�����������ã���1 000 mL��Ͳ���ѳ�����ˮ����ʼʵ��ʱ������Ҫ______��
��2��a�缫����Ϊ________����缫��ӦʽΪ______________��b�缫����Ϊ________����缫��ӦʽΪ______________��
��3�� ����Ͳ���ռ�672 mL����ʱ(�����㵽��״����)��ͨ�����ߵĵ��ӵ����ʵ���Ϊ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ��������������ԭ�����͵���( )
A. ��H2����������HI��ɵ�ƽ����ϵ��ѹ����ɫ����
B. ����ɫ����ˮ���պ���ɫ��dz
C. ��ѹ��ʹ�����SO2ת��ΪSO3
D. �ں���Fe(SCN)2+�ĺ�ɫ��Һ�м����ۣ����ã���Һ��ɫ��dz����ȥ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I.�����仯������;�dz��㷺����֪Ǧ(Pb)���ͬ������Pb��Si��3�����Ӳ㡣�ش��������⣻
(1)��λ��Ԫ�����ڱ��ĵ�______����______�塣Pb��Si��ԭ�Ӱ뾶______(������������С��)
(2)������PbSO4����CH3COONH4��Һ���Ƶ�(CH3COO)2Pb[(CH3COO)2Pb������ˮ]�������ķ�ӦΪPbSO4+2CH3COONH4=(CH3COO)2Pb+(NH4)2SO4��˵��(CH3COO)2Pb��______(����ǿ����������)����ʡ�
(3)Ǧ���س�ŵ�ԭ��ΪPb+PbO2+2H2SO4![]() 2PbSO4+2H2O�����й�����B��Ϊ______(�����ŵ������������)��
2PbSO4+2H2O�����й�����B��Ϊ______(�����ŵ������������)��
(4)��PbO2���������ữ��Mn(NO3)2��Һ�н������Һ���Ϻ�ɫ����ÿ��Ӧ1 mol Mn(NO3)2ת��____________mol���ӡ�
��.Ԫ�ظ�(Cr)����Ȼ����Ҫ��+3�ۺ�+6�۴��ڡ���ҵ�����ø�����(FeO��Cr2O3)ұ�����Ĺ���������ͼ��ʾ��

(1)ˮ��IҪ��ý���Һ�IJ�����______��
(2)������Cr(OH)3���ܶȻ�Ksp=1��10-32����ҪʹCr3+��ȫ��������pHΪ______[c(Cr3+)����10-5 mol/L����Ϊ��ȫ����]��
(3)�Ը�����(Na2CrO4)Ϊԭ�ϣ��õ绯ѧ�����Ʊ��ظ�����Na2Cr2O7)��ʵ��װ����ͼ��ʾ(��֪��2CrO42-+2H+=C2O72-+H2O)��
�����ĵ缫��ӦʽΪ______��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ƿ�ռ��������������������壬Ȼ���䵹����ˮ���С��ֱ���ͨ������O2��Cl2����ͼ��ʾ��һ��ʱ���D��Eװ�õļ���ƿ�г�����Һ��Fװ�õļ���ƿ�л�������ʣ�ࡣ(����ƿ��Һ�岻��ɢ)

(1)д��װ��E�з�Ӧ�����ӷ���ʽ��__________________________________________
(2)�����ʵ�������£�����Ħ�����ΪaL��mol��1����װ��D�ļ���ƿ��������Һ���ʵ����ʵ���Ũ��Ϊ____________��������Ӧ�ķ���ʽ____________________________��
(3)ͨ������ǰ��Fװ�õ�ˮ����μӼ�����ɫʯ����Һ���۲쵽��������_____________��ͨ���������ܹ۲쵽��ʵ��������_________________________________________��д����Ӧ���ܻ�ѧ����ʽ��_________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ�����Ʊ�������װ�ã����£����ش��������⣻

(1)����A �� B����___________ ��____________
(2)д��B�з�����Ӧ�����ӷ���ʽ_____________________________________
(3)װ��C������__________________________________________
(4)װ��D������________________________________________________
(5)����E�������Ѿ��ռ����ķ���_________________________________________
(6)д��F�з����ķ�Ӧ����ʽ______________________________________________
(7)ÿ����0.5mol����ת��________________������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Co(H2PO2)2�㷺���ڻ�ѧ���ܣ��Խ���Co��NaH2PO2Ϊԭ�ϣ��������ҵ������۵�ⷨ�Ʊ���ԭ����ͼ������������ȷ����

A. bΪ�����ӽ���Ĥ
B. ͨ���ԭ���ҵ�H2PO2���Ʒ��Ǩ�ƣ���������ҺpH����
C. ʯī�缫��ӦΪ4OH4e![]() O2��+2H2O
O2��+2H2O
D. ������1 mol Co(H2PO2)2ʱ��������״��������11.2 L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1�����ײ��϶������ѣ�TiO2�����кܸߵĻ�ѧ���ԣ��������������Ĵ�������ҵ�϶������ѵ��Ʊ��ǣ�
���Ͽ�Ƭ | ||
���� | �۵� | �е� |
SiCl4 | -70�� | 57.6�� |
TiCl4 | -25�� | 136.5�� |
I. �������Ľ��ʯ����Ҫ�ɷ�TiO2����Ҫ����SiO2����̼�ۻ��װ���Ȼ�¯�У��ڸ�����ͨ��Cl2��Ӧ�Ƶû���SiCl4���ʵ�TiCl4��
II. ��SiCl4���룬�õ�������TiCl4��
III. ��TiCl4�м�ˮ�����ȣ�ˮ��õ�����TiO2��xH2O��
IV. TiO2��xH 2O���·ֽ�õ�TiO2��
��TiCl4��SiCl4�ڳ����µ�״̬��________��II������ȡ�IJ���������_______��
��III�з�Ӧ�Ļ�ѧ����ʽ��____________________________________________��
����IV��ʵ������ɣ�Ӧ��TiO2��xH2O����________����������ţ��м��ȡ�

��2�����ݷ�ˮ�������к����ʵIJ�ͬ����ҵ���ж��ַ�ˮ�Ĵ���������

�ٷ�ˮI������CO2���������ӷ���ʽ��________________��
�ڷ�ˮ��������������ʵ���з��ַ�ˮ�е�c(HCO![]() )Խ��ˮЧ��Խ�ã�������Ϊ______________��
)Խ��ˮЧ��Խ�ã�������Ϊ______________��
�۷�ˮIII�еĹ�Ԫ�ش�������ת�����ڿո�������Ӧ�Ļ�ѧʽ����Hg2++______=CH3Hg++H+���ҹ��涨��Hg2+���ŷű����ܳ���0.05 mg��L����ij�����ŷŵķ�ˮ1 L�к�Hg2+ 3��10-7mo1���Ƿ�ﵽ���ŷű�__����ǡ�����
�ܷ�ˮ������C12����CN����CO2��N2�����μӷ�Ӧ��C12 ��CN-�����ʵ���֮��Ϊ5�U2����÷�Ӧ�����ӷ���ʽΪ__________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com