
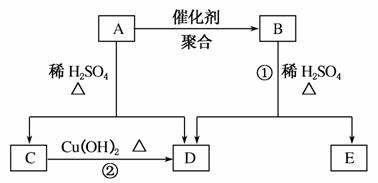
»ÆŗĻĪļAĻą¶Ō·Ö×ÓÖŹĮæĪŖ86£¬Ģ¼µÄÖŹĮæ·ÖŹżĪŖ55.8%£¬ĒāĪŖ7.0%£¬ĘäÓąĪŖŃõ”£AµÄĻą¹Ų·“Ó¦ČēĻĀĶ¼ĖłŹ¾£ŗ
ŅŃÖŖR—CH===CHOH(Ļ©“¼)²»ĪČ¶Ø£¬ŗÜæģ×Ŗ»ÆĪŖR—CH2CHO”£
øł¾ŻŅŌÉĻŠÅĻ¢»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)AµÄ·Ö×ÓŹ½ĪŖ________________£»
(2)·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½ŹĒ________________________________________________________________________£»
(3)AµÄ½į¹¹¼ņŹ½ŹĒ________________£»
(4)·“Ó¦¢ŁµÄ»Æѧ·½³ĢŹ½ŹĒ________________________________________________________________________£»
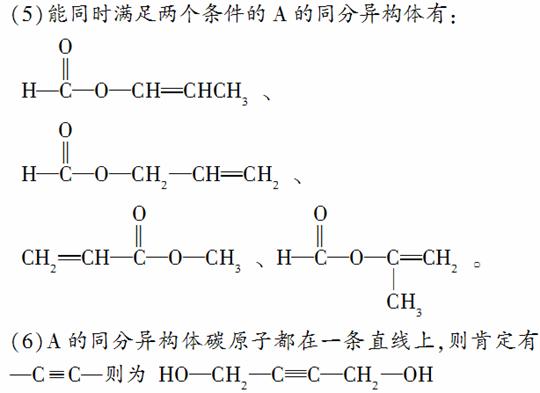
(5)AÓŠ¶ąÖÖĶ¬·ÖŅģ¹¹Ģ壬Š“³öĖÄøöĶ¬Ź±Āś×ć(ⅰ)ÄÜ·¢ÉśĖ®½ā·“Ó¦(ⅱ)ÄÜŹ¹äåµÄĖÄĀČ»ÆĢ¼ČÜŅŗĶŹÉ«Į½øöĢõ¼žµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½£ŗ____________”¢____________”¢____________”¢____________£»
(6)AµÄĮķŅ»ÖÖĶ¬·ÖŅģ¹¹Ģ壬Ęä·Ö×ÓÖŠĖłÓŠĢ¼Ō×ÓŌŚŅ»ĢõÖ±ĻßÉĻ£¬ĖüµÄ½į¹¹¼ņŹ½ĪŖ________________”£
½āĪö””(1)AµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ86£¬Ģ¼µÄÖŹĮæ·ÖŹżĪŖ55.8%£¬ŌņĢ¼µÄÖŹĮæĪŖ48£¬ĘäŌ×ÓøöŹżĪŖ4£¬ĒāµÄÖŹĮæ·ÖŹżĪŖ7.0%£¬ŌņĒāµÄŌ×ÓøöŹżĪŖ6£¬“Ó¶ųæÉÖŖŃõµÄŌ×ÓøöŹżĪŖ2£¬ĖłŅŌAµÄ·Ö×ÓŹ½ĪŖC4H6O2”£
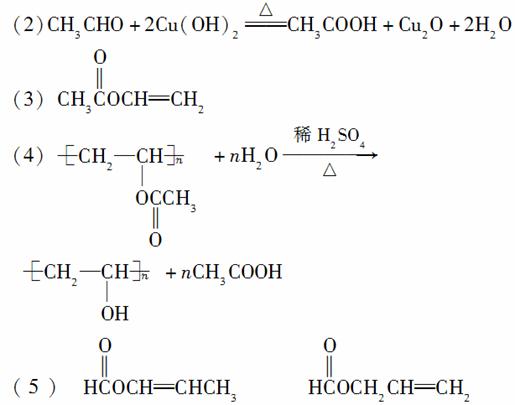
(2)AÄÜŌŚĻ”H2SO4¼ÓČČĢõ¼žĻĀ·“Ó¦£¬ĖµĆ÷AÖŠŗ¬ÓŠõ„»ł£»CÄÜÓėCu(OH)2ŌŚ¼ÓČČĢõ¼žĻĀ·“Ó¦µĆD£¬ŌņCŗ¬ÓŠČ©»ł£¬DĪŖōČĖį£¬ŌŁÓÉŅŃÖŖæɵĆCĪŖCH3CHO£¬DĪŖCH3COOH£¬ĖłŅŌ·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½ŹĒ£ŗCH3CHO£«2Cu(OH)2 CH3COOH£«Cu2O”ż£«2H2O”£
CH3COOH£«Cu2O”ż£«2H2O”£

“š°ø””(1)C4H6O2



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ā±“śĢžÄÜ·¢ÉśĻĀĮŠ·“Ó¦£ŗ2CH3CH2Br£«2Na”Ŗ”śCH3CH2CH2CH3£«2NaBr”£ĻĀĮŠÓŠ»śĪļæÉŗĻ³É»·±ūĶé(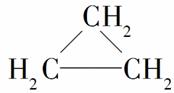 )µÄŹĒ(””””)
)µÄŹĒ(””””)
A£®CH3CH2CH2Br B£®CH3CHBrCH2Br
C£®CH2BrCH2CH2Br D£®CH3CHBrCH2CH2Br
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ·“Ó¦ĖłµĆČÜŅŗÖŠŅ»¶ØÖ»ŗ¬Ņ»ÖÖČÜÖŹµÄŹĒ £Ø £©
A£®ĻņNaOHČÜŅŗÖŠĶØČėCO2 B£®ĻņCa£ØOH£©2ČÜŅŗÖŠĶØČėCl2
C£®ĻņÕōĮóĖ®ÖŠ¼ÓČėÉŁĮ潚ŹōNa D£®ĻņA12£ØSO4£©3ČÜŅŗÖŠµĪČėBa£ØOH£©2ČÜŅŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
3¼×»łĪģĶéµÄŅ»ĀČ“ś²śĪļÓŠ(²»æ¼ĀĒĮ¢ĢåŅģ¹¹)(””””)
A£®3ÖÖ B£®4ÖÖ
C£®5ÖÖ D£®6ÖÖ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠø÷×éĢžµÄ»ģŗĻĪļ£¬Ö»ŅŖ×ÜÖŹĮæŅ»¶Ø£¬°“ČĪŅā±ČĄż»ģŗĻ£¬ĶźČ«Č¼ÉÕŗóÉś³ÉµÄCO2ŗĶH2O¶¼ŹĒŗćĮæµÄŹĒ(””””)
A£®C2H2”¢C2H4 B£®C2H4”¢C4H6
C£®C2H6”¢C3H6 D£®C6H6”¢C2H2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijӊ»śĪļµÄ½į¹¹¼ņŹ½ČēĶ¼£¬ĻĀĮŠ½įĀŪÕżČ·µÄŹĒ (””””)”£

A£®øĆÓŠ»śĪļ·Ö×ÓŹ½ĪŖC13H16
B£®øĆÓŠ»śĪļŹōÓŚ±½µÄĶ¬ĻµĪļ
C£®øĆÓŠ»śĪļ·Ö×ÓÖŠÖĮÉŁÓŠ4øöĢ¼Ō×Ó¹²Ö±Ļß
D£®øĆÓŠ»śĪļ·Ö×ÓÖŠ×ī¶ąÓŠ13øöĢ¼Ō×Ó¹²Ę½Ćę
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠŹµŃéÄÜ»ńµĆ³É¹¦µÄŹĒ (””””)”£
A£®ÓĆäåĖ®æɼų±š±½”¢CCl4”¢¼ŗĻ©
B£®¼ÓÅØäåĖ®£¬Č»ŗó¹żĀĖæɳżČ„±½ÖŠÉŁĮæ¼ŗĻ©
C£®±½”¢äåĖ®”¢Ģś·Ū»ģŗĻÖĘ³Éäå±½
D£®æÉÓĆ·ÖŅŗĀ©¶··ÖĄė¼ŗĶéŗĶ±½µÄ»ģŗĻĪļ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŌäåŅŅĶéĪŖŌĮĻÖĘČ”1,2¶žäåŅŅĶ飬ĻĀĮŠ×Ŗ»Æ·½°øÖŠ×īŗĻĄķµÄŹĒ(””””)
A£®CH3CH2Br CH2BrCH2Br
CH2BrCH2Br
B£®CH3CH2Br CH2BrCH2Br
CH2BrCH2Br
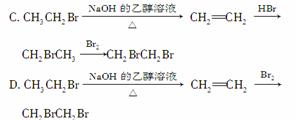
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖt ”ꏱAgClµÄKsp£½4”Į10£10£¬ŌŚt ”ꏱ£¬Ag2CrO4ŌŚĖ®ÖŠµÄ³ĮµķČܽāĘ½ŗāĒśĻßČēĶ¼ĖłŹ¾”£ĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ (””””)”£
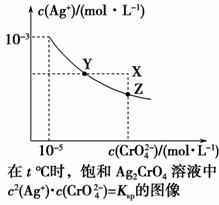
A£®ŌŚt ”ꏱ£¬Ag2CrO4µÄKspĪŖ1”Į10£11
B£®ŌŚ±„ŗĶČÜŅŗÖŠ¼ÓČėK2CrO4(s)æÉŹ¹ČÜŅŗÓÉYµćµ½Zµć
C£®ŌŚt ”ę£¬Ag2CrO4(s)£«2Cl£(aq)2AgCl(s)£«CrO (aq)Ę½ŗā³£ŹżK£½6.25”Į107
(aq)Ę½ŗā³£ŹżK£½6.25”Į107
D£®ŌŚt ”ꏱ£¬ŅŌ0.001 mol”¤L£1 AgNO3ČÜŅŗµĪ¶Ø20 mL 0.001 mol”¤L£1 KClŗĶ0.001 mol”¤L£1µÄK2CrO4µÄ»ģŗĻČÜŅŗ£¬CrO ĻČ³Įµķ
ĻČ³Įµķ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com