
| ĒāŃõ»ÆĪļ | Fe(OH)3 | Al(OH)3 | Mg(OH)2 |
| æŖŹ¼³ĮµķpH | 1.5 | 3.3 | 9.4 |
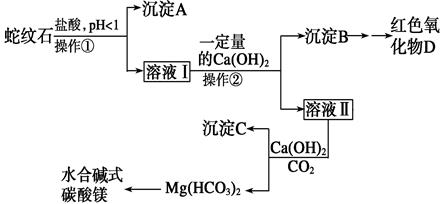
| A£®Š”ÓŚ1.5 | B£®1.5”«3.3 |
| C£®7”«8 | D£®“óÓŚ9.4 |


 ĆūŠ£Į·æ¼¾ķĘŚÄ©³å“Ģ¾ķĻµĮŠ“š°ø
ĆūŠ£Į·æ¼¾ķĘŚÄ©³å“Ģ¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
 ²Ł×÷ŹĒ ”£
²Ł×÷ŹĒ ”£ ÉÕĘæÄŚ²śÉśµÄĘųĢåÖ÷ŅŖÓŠ£ØŠ“»ÆѧŹ½£© ”£
ÉÕĘæÄŚ²śÉśµÄĘųĢåÖ÷ŅŖÓŠ£ØŠ“»ÆѧŹ½£© ”£ Į¬½ÓĖ³ŠņŹĒ£ØĢī×ÖÄø£©£Ø””£©”ś£Ø””
Į¬½ÓĖ³ŠņŹĒ£ØĢī×ÖÄø£©£Ø””£©”ś£Ø”” £©”ś£Ø””£©”śE
£©”ś£Ø””£©”śE²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
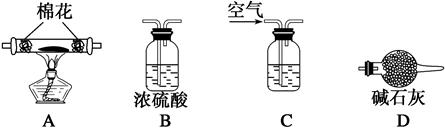
| A£®Ö»ÓŠ¢ŁŗĶ¢Ś“¦ |
| B£®Ö»ÓŠ¢Ś“¦ |
| C£®Ö»ÓŠ¢ŚŗĶ¢Ū“¦ |
| D£®Ö»ÓŠ¢Ś”¢¢Ū”¢¢Ü“¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
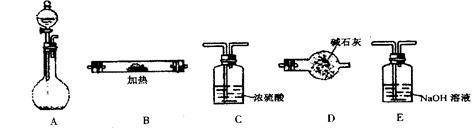
| A£®ÓĆĘōĘÕ·¢ÉśĘ÷ÖĘČ”£¬ÓĆÅÅĖ®·ØŹÕ¼Æ |
| B£®ÓĆĘōĘÕ·¢ÉśĘ÷ÖĘČ”£¬ÓĆĻņĻĀÅÅæÕĘų·ØŹÕ¼Æ |
| C£®Óė¼ÓČČ·Ö½āKClO3ÖĘŃõĘųµÄ×°ÖĆĻąĶ¬£¬ÓĆĻņĻĀÅÅæÕĘų·ØŹÕ¼Æ |
| D£®Óė¼ÓČČ·Ö½āKClO3ÖĘŃõĘųµÄ×°ÖĆĻąĶ¬£¬ÓĆĻņÉĻÅÅæÕĘų·ØŹÕ¼Æ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
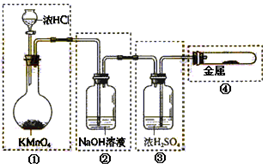
| A£®“Ė·ØµÄÓŵćÖ®Ņ»ŹĒŌĮĻĄ“Ō“·įø» |
| B£®½ųŠŠ¢Ł¢Ś¢Ū²½ÖčµÄÄæµÄŹĒ“Óŗ£Ė®ÖŠĢįČ”ĀČ»ÆĆ¾ |
| C£®µŚ¢Ü²½µē½āÖĘĆ¾ŹĒÓÉÓŚĆ¾ŹĒŗÜ»īĘĆµÄ½šŹō |
| D£®ŅŌÉĻÖĘČ”Ć¾µÄ¹ż³ĢÖŠÉę¼°µÄ·“Ó¦ÓŠ·Ö½ā·“Ó¦”¢»ÆŗĻ·“Ó¦ŗĶÖĆ»»·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
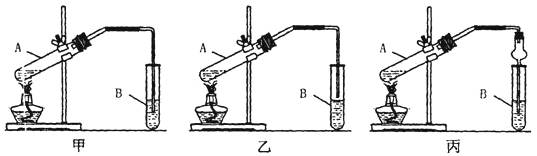
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
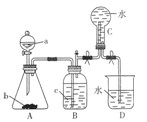

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
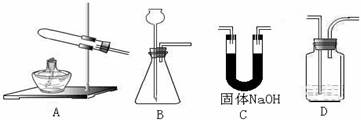
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com