
 ��
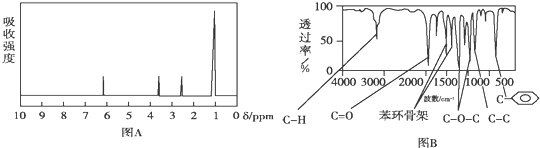
�� ���� ��1������A�ĺ˴Ź������������շ���Ŀ�ж��京�еĵ�Ч��ԭ����Ŀ��
��2������A����Է���������A������C��H����������ȷ��A�ķ���ʽ��
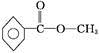
��3������ͼBȷ��A�����к��еĹ����ţ�Ȼ���ж������ͣ�
��4������A�ķ���ʽ����˴Ź���������ȷ��A�����к���1������
��5���������Ϸ���ȷ��A�Ľṹ��ʽ��
��� �⣺��1������A�ĺ˴Ź�������֪��A��4���壬��A����4����ԭ�ӣ�
�ʴ�Ϊ��4��
��2��A������C��H��Oԭ����Ŀ֮��Ϊ��N��C����N��H����N��O��=$\frac{70.59%}{12}$��$\frac{5.88%}{1}$��$\frac{1-70.59%-5.88%}{16}$=4��4��1��
��A��ʵ��ʽΪC4H4O����A�ķ���ʽΪ��C4H4O��n����Mr��A��=68n=136����ã�n=2�������ʽΪC8H8O2��
�ʴ�Ϊ��C8H8O2��
��3����ͼB֪��A���б�����ռ6��Cԭ�ӣ�������C=O��C-O-C��C-C��C-H������C=O��C-O-C�����Ϊ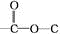 �����Ը�����Ϊ���࣬
�����Ը�����Ϊ���࣬
�ʴ�Ϊ�����ࣻ
��4�����������3���Ľ������ٽ��ͼA����4����ԭ�ӣ��������Ϊ1��1��1��3��������-CH3����ԭ����Ϊ3������ֻ����һ��-CH3������A�ķ�����ֻ��һ����������Ϊbc���ʴ�Ϊ��bc��
��5���������Ϸ�����֪��A�Ľṹ��ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼�����л������ʽ���ṹ��ʽ��ȷ������Ŀ�Ѷ��еȣ���ȷ�˴Ź������ס�������ĺ���Ϊ���ؼ���ע�����������غ㶨����ȷ���л������ʽ�е�Ӧ�ã�����������ѧ���ķ���������������


 ���ʿ��ÿ��ֳɳ�ϵ�д�
���ʿ��ÿ��ֳɳ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �״� | B�� | �Ҵ� | C�� | ���� | D�� | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����Ͷ��黥Ϊͬϵ�� | |
| B�� | ��ϩ�Ͷ�ϩ��Ϊͬϵ�� | |
| C�� | ����Ͷ�����̼Ԫ�ص�����������ͬ | |
| D�� | ��ϩ�Ͷ�ϩ��̼Ԫ�ص�����������ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������̼����ˮ | |
| B�� | �Ȼ�������ˮ | |
| C�� | ˮ�������� | |
| D�� | ú������˹����Ҫ�ɷ��Ǽ��飩����ը |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ����Ӧ�ܵõ���ѧʽΪC7H5O3Na���ǣ�������
����Ӧ�ܵõ���ѧʽΪC7H5O3Na���ǣ�������| A�� | NaHCO3��Һ | B�� | Na2CO3��Һ | C�� | NaOH��Һ | D�� | NaCl��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | BaSO4��BaCO3��������ˮ���������������͡� | |
| B�� | �ִ�����Ǻ�������������Ҫ�����˵绯ѧ��ʴ | |
| C�� | ��ʯ��ˮ������������֪�ı�ʯ�����仯ѧ�ɷֲ�ͬ | |
| D�� | �������������������ھ�ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
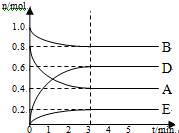 ij�¶�T���£���ij���̶��ݻ�Ϊ2.0L���ܱ������ڣ���ʱ����ͼ��ʾ������Ӧ������A��B��DΪ���壬EΪ���壮
ij�¶�T���£���ij���̶��ݻ�Ϊ2.0L���ܱ������ڣ���ʱ����ͼ��ʾ������Ӧ������A��B��DΪ���壬EΪ���壮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����Բⶨijδ֪Ũ�ȵĴ�����Һ�д���ĵ��볣��Ka��Ӧ����ʵ���������Լ�������ֽ��Ϊ���к͵ζ�ʵ�顢pH��ֽ | |
| B�� | ��0.1mol/L��NaOH��Һ��0.5mol/L��CuSO4��Һ���������Ƶ�������ͭ����Һ�����ڼ�����ѿ���ǻ�ԭ���� | |
| C�� | ���ܱ������м���1.5mol H2��0.5mol N2��ַ�Ӧ�ɵõ�NH3������ΪNA | |
| D�� | ��״���£�33.6L���ȼ����к�����ԭ�ӵ���ĿΪ3NA |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com