
��֪��ϡ��Һ��±����ˮ�⡣�����廯����C�ķ���ʽΪC9H9OCl��C�������ж���������������һ�ȴ���ֻ�ж��֣���˴Ź�������ͼ����������շ壬���շ�����֮��Ϊ1��2��2��2��2����һ�������£�������C�ɷ�����ͼ��ʾ��ת����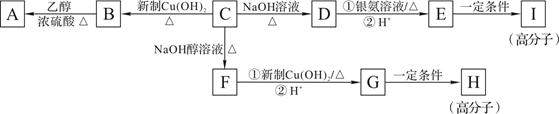
��1��B��A�ķ�Ӧ������_______��H�Ľṹ��ʽ��________��
��2��C������������_____��̼ԭ�ӹ��棬�京�������ŵ����� ��
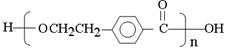
��3��д�����л�ѧ����ʽ��D��������Һ��Ӧ_________��E��I________��
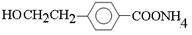
��4��D��һ��ͬϵ��W����ʽΪC8H8O2�����������������W��ͬ���칹�干��_____�֡���������
���ڷ����廯�������FeCl3��Һ������ɫ ��1 mol W���뺬2 mol NaOH����Һ��Ӧ
��15�֣���1��ȡ����Ӧ��������Ӧ����1�֣��� (2��) ��
(2��) ��
��2��8 ��2�֣���ȩ����2�֣���
��3��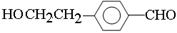 ��2Ag(NH3)OH
��2Ag(NH3)OH
 +2Ag����3NH3��H2O(3��)��
+2Ag����3NH3��H2O(3��)��
n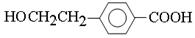
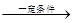
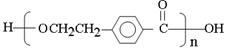 ��(n-1)H2O (3��)
��(n-1)H2O (3��)
��4��4��2�֣�
�������������C�����ܺ����Ƶ�������ͭ����Һ��Ӧ��˵��C�����к���ȩ����ϡ��Һ��±����ˮ�⣬��C�ܺ�����������Ӧ����˵��C�����е���ԭ�Ӳ������ڱ����ϵġ�C�������ж���������������һ�ȴ���ֻ�ж��֣���˵��2�������Ƕ�λ�ġ���˴Ź�������ͼ����������շ壬���շ�����֮��Ϊ1��2��2��2��2�����C�Ľṹ��ʽ��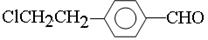 ��C����B��ȩ����������Ӧ����B�Ľṹ��ʽ��
��C����B��ȩ����������Ӧ����B�Ľṹ��ʽ��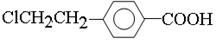 ��B�к����Ȼ����ܺ��Ҵ�����������Ӧ����A����A�Ľṹ��ʽ��
��B�к����Ȼ����ܺ��Ҵ�����������Ӧ����A����A�Ľṹ��ʽ�� ��C����D����ԭ�ӵ�ˮ�ⷴӦ����D�Ľṹ��ʽ��
��C����D����ԭ�ӵ�ˮ�ⷴӦ����D�Ľṹ��ʽ�� ��D����������Ӧ�ữ������E����E�Ľṹ��ʽ��
��D����������Ӧ�ữ������E����E�Ľṹ��ʽ��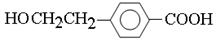 ��E�к����ǻ����Ȼ����ܷ������۷�Ӧ�����ɸ߷��ӻ�����I����I�Ľṹ��ʽ��
��E�к����ǻ����Ȼ����ܷ������۷�Ӧ�����ɸ߷��ӻ�����I����I�Ľṹ��ʽ��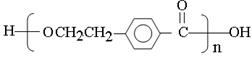 ��C���������ƵĴ���Һ�з�����ԭ�ӵ���ȥ��Ӧ����F����F�Ľṹ��ʽ��
��C���������ƵĴ���Һ�з�����ԭ�ӵ���ȥ��Ӧ����F����F�Ľṹ��ʽ��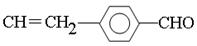 ��F�е��ǻ������������Ȼ�����G�Ľṹ��ʽ��
��F�е��ǻ������������Ȼ�����G�Ľṹ��ʽ��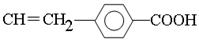 ��G�к���̼̼˫�����ܷ����Ӿ۷�Ӧ���ɸ߷��ӻ�����H����H�Ľṹ��ʽ��
��G�к���̼̼˫�����ܷ����Ӿ۷�Ӧ���ɸ߷��ӻ�����H����H�Ľṹ��ʽ�� ��
��
��1��B��A�ķ�Ӧ������������Ӧ����ȡ����Ӧ����H�Ľṹ��ʽ��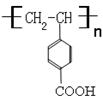 ��
��
��2�����ڱ�����ƽ���ͽṹ����C������������8��̼ԭ�ӹ��棬�京�������ŵ�����ȩ����
��3��D��������Һ��Ӧ�Ļ�ѧ����ʽ��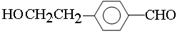 ��2Ag(NH3)OH
��2Ag(NH3)OH
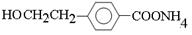 +2Ag����3NH3��H2O(3��)��
+2Ag����3NH3��H2O(3��)��
E��I�����۷�Ӧ����Ӧ�Ļ�ѧ����ʽ��n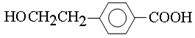
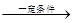
 ��(n-1)H2O��
��(n-1)H2O��
��4�������ڷ����廯���˵�����б���������FeCl3��Һ������ɫ��˵�������з��ǻ�����1 mol W���뺬2 mol NaOH����Һ��Ӧ��˵��������������ˮ����ǻ������ڱ����ϣ����Կ��ܵĽṹ��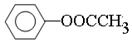 ��
��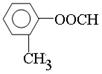 �����м�λ�Ͷ�λ����������4�֡�
�����м�λ�Ͷ�λ����������4�֡�
���㣺�����л�����ƶϣ��л���Ӧ���͡��������Լ��л��ﹲ����жϣ�ͬ���칹���ж��Լ��л���Ӧ����ʽ����д��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
PTT�ǽ�������Ѹ�ٷ�չ���������������Ծ������ϣ������������ܣ�����Ϊ�������ϡ���֯��ά�͵�̺�Ȳ��϶��õ��㷺Ӧ�á���ϳ�·�߿����Ϊ��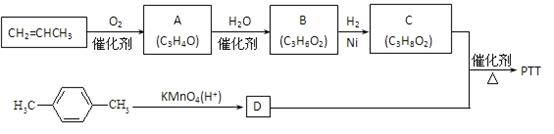
����A��B��C��Ϊ��״�����A�ܷ���������Ӧ��C�в�������1mol C���������Ʒ�Ӧ����22��4 L H2����״��������ش��������⣺
��1��A�����������ŵ�����Ϊ_________��B�Ľṹ��ʽΪ________��
��2��������C��D��Ӧ����PTT�Ļ�ѧ����ʽΪ________����Ӧ����Ϊ________��
��3������ʽΪC4H6O����A��Ϊͬϵ���ͬ���칹����________�֡�
��4���벹������������CH2=CHCH3Ϊ��Ҫԭ�ϣ����Լ����ã��Ʊ�CH3CH��OH��COOH
�ĺϳ�·������ͼ����ע����Ӧ��������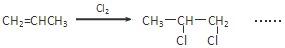
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
ij��ˮ�����������ά������Ҫ�Ļ���ԭ�ϡ����ǵĺϳ�·����ͼ��ʾ��
��֪����A��C��H��O����Ԫ����ɣ���Է�������Ϊ32��
��RCOOR�䣫R��OH RCOOR�士R��OH(R��R�䡢R���������)��
RCOOR�士R��OH(R��R�䡢R���������)��
��ش��������⣺
(1)A�Ľṹ��ʽ��____________________��
(2)B�еĹ�����������______________________��
(3)D��E�ķ�Ӧ������______________��
(4)�������뻯����M��Ӧ�Ļ�ѧ����ʽ��_________________________________
��G��������ά�Ļ�ѧ����ʽ��____________________________
(5)E��������___________��
(6)G��ͬ���칹���ж��֣��������������Ĺ���_____�֡�
�ٱ�����ֻ������ȡ������
��1 mol��������������NaHCO3��Һ��Ӧ����2 mol CO2��
(7)д���� �ϳ�
�ϳ� ������ͼ(ע����Ӧ����)��
������ͼ(ע����Ӧ����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��16�֣���/̼��Ч��ɫ��Suzuki ����ż����Ӧ�ǽ������л��ϳɵ��ȵ�֮һ���練Ӧ�٣�
I II
���������ɻ�����III�ϳɣ�
��1���������ķ���ʽΪ �����еĹ���������Ϊ ��
��2����������Ľṹ��ʽΪ ��
��3���������������Cu(OH)2����Һ��Ӧ�Ļ�ѧ����ʽΪ ��ע����������
��4�����������һ��ͬ���칹�����FeCl3��Һ����ɫ���˴Ź����������֮��Ϊ2��2��2��1��������Ľṹ��ʽΪ _________ ��д���������������NaOHˮ��Һ��Ӧ�Ļ�ѧ����ʽΪ ��ע����������
��5�������� �뻯����
�뻯���� ��һ�������ɷ������Ʒ�Ӧ�ٵķ�Ӧ�������Ľṹ��ʽΪ ��
��һ�������ɷ������Ʒ�Ӧ�ٵķ�Ӧ�������Ľṹ��ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��֪��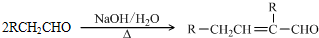
ˮ������EΪ�������ռ������������Ʒ�ɹ˪��E��һ�ֺϳ�·�����£�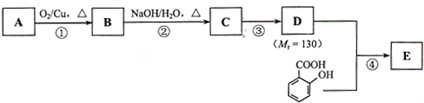
��ش��������⣺
��1��һԪ��A��������������ԼΪ21.6%����A�ķ���ʽΪ ���ṹ������ʾAֻ��һ������A������Ϊ ��
��2��B�������Ƶ�Cu(OH)2������Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3��C�� �ֽṹ����һ��ȡ��������C�����������ţ���ʹ�õ��Ⱥ�˳��д�������Լ� ��
��4���ڢ۵ķ�Ӧ����Ϊ ��D���������ŵ�����Ϊ ��
��5��д��ͬʱ��������������ˮ��������ͬ���칹��Ľṹ��ʽ�� ��
A�������к���6��̼ԭ����һ������
B�����������������Ű���ˮ������еĹ�����
��6���ڢܲ��ķ�Ӧ����Ϊ ��д��E�Ľṹ��ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��13�֣���֪����A�����ԣ���������¿�ͼ�ش�����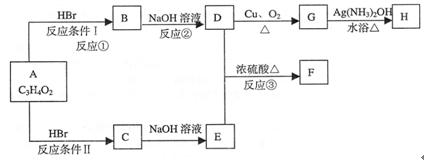
FΪ��ԭ����ɵĻ�״�ṹ
��1��A�Ľṹ��ʽΪ ��2�֣�
��2���٢ڢ۵ķ�Ӧ���ͷֱ�Ϊ �� �� ��3�֣�
��3��������B�к��й����ŵ������� ��2�֣�
��4��C����E�Ļ�ѧ����ʽ ��2�֣�
��5��G����H�Ļ�ѧ����ʽ ��2�֣�
��6��д��C��ͬ���칹���������������ʵĽṹ��ʽ ��
����д2�֣���2�֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�����������͡����ס���Ч������ʧ��ҩ�����ڶ�������ʧ���ķ��Ρ��ṹΪ��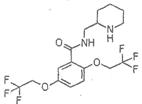 �������������ᣨ
�������������ᣨ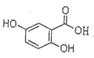 ��Ϊԭ�Ϻϳɣ��ϳɵķ�������ͼ��
��Ϊԭ�Ϻϳɣ��ϳɵķ�������ͼ��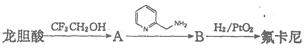
�ش��������⣺
��1��д��һ���������к��еĺ�������������______________��
��2��A�Ľṹ��ʽ��____________________________________��
��3��A��B�ķ�Ӧ������______________��
��4����������״���Ӧ����������������仯ѧ����ʽ��______________��
��5���������������������������ͬ���칹�干��_______�֣�
�ٱ�������3��ȡ����������2��Ϊ�ǻ����ڱ�����ֻ�������⣻���������ࡣ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�л���A��G��ת����ϵ����ͼ��ʾ������A��һԪ��״�������˴Ź���������ֻ��1���壻F�ĺ˴Ź�����������3���壬�������Ϊ2��2��3��G��һ�ֺϳ�����֬��һ����Ҫԭ�ϡ�
��֪��(RΪ����) ��
��RCOOH RCH2OH
RCH2OH
��ش��������⣺
��1��C�����������ŵ������� ����Ӧ�ܵķ�Ӧ������___________________��
��2����E��һ�������·������۷�Ӧ���ɸ߷��ӻ����д���������ֵĽṹ��ʽ��
_____________________________��_____________________________��
��3����Ӧ�ڵĻ�ѧ����ʽΪ ��
��4����Ӧ�Ļ�ѧ����ʽΪ________________________________________________________��
��5���л���Y��E��Ϊͬ���칹�壬���Ҿ�����ͬ�Ĺ������������Ŀ��д�����з���������Y�Ľṹ��ʽ��_____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ʡ��һ��ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�����й�0.2mol��L��1 BaCl2��Һ��˵������ȷ����( )
A��500mL��Һ��Cl������Ũ��Ϊ0.2mol��L��1
B��500mL��Һ��Ba2������Ũ��Ϊ0.2mol��L��1
C��500mL��Һ��Cl����������Ϊ0.2NA
D��500mL��Һ��Ba2����Cl����������Ϊ0.3NA
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com