
ijУ��ѧС��ѧ��������ͼ����װ�ý��С�����ˮ��Ӧ����ʵ�飬�����ò����һ����ȡ
FeCl3��6H2O���塣��ͼ�мгּ�β������װ�þ�����ȥ��

��1��װ��B�з�����Ӧ�Ļ�ѧ����ʽ��___________________________��
��2��װ��B�е�������________________________________________��
��3��ֹͣ��Ӧ����B����ȴ��ȡ���еĹ��壬�������ϡ�����ַ�Ӧ�����ˡ�����������Һ��Fe3+�IJ���������________________________��
��4����С��ѧ������������Һ��ȡFeCl3��6H2O���壬����������£�
��Һ FeCl3��Һ
FeCl3��Һ FeCl3��6H2O����
FeCl3��6H2O����
�ٲ������ͨ��Cl2��������____________________________________��
�ڲ�����FeCl3ϡ��Һ�еõ�FeCl3��6H2O�������Ҫ����������
_____________________________________________________________��
�۸��������豣�������������Ҫԭ���ǣ�������ӷ���ʽ��Ҫ˵����
_____________________________________________________________��
���𰸡�
��1��3Fe+4H2O(g) Fe3O4+4H2
Fe3O4+4H2
��2����ɫ�����죬�Ҷ˹ܱ���ˮ��
��3��ȡ������Һ�����뼸��KSCN��Һ���۲���Һ�Ƿ���ɫ
��4���ٽ�Fe2+������Fe3+
�ڼ���Ũ������ȴ�ᾧ������
��Fe3++3H2O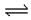 Fe(OH)3+3H+��������������FeCl3ˮ��
Fe(OH)3+3H+��������������FeCl3ˮ��
��������װ���и����ֵ����ü����ܷ����ķ�Ӧ��
A.����ˮ���������Ƭ���ֹ���е�����
B.ˮ�����ڸ�������Fe��Ӧ��3Fe��4H2O Fe3O4��4H2
Fe3O4��4H2
C.���塢��ȴ������ʹ�����е�H2O��������
D.�������ɵ�H2
E.H2�ڼ��������»�ԭCuO��H2��CuO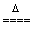 Cu��H2O
Cu��H2O
Fe3O4�������Fe3O4��8HCl==2FeCl3��FeCl2��4H2O������Fe3���ܺ�SCN����Ӧ������Ѫ��ɫ��Һ��Fe3����3SCN��==Fe��SCN��3��
����Һ��ͨ��Cl2�ܽ�Fe2��ת���Fe3����2Fe2����Cl2==2Fe3����2Cl����


 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ��ȤС���ijƷ��������Ħ�����ɷּ��京����������̽����
������ϣ�������Ħ������̼��ơ�����������ɣ������������ɷ���������ʱ���������ɡ�
��.Ħ���������������Ķ��Լ���
ȡ����������Ʒ����ˮ�ɷֽ��衢���ˡ�
��1���������м������NaOH��Һ�����ˡ�����������NaOH��Һ��Ӧ�����ӷ���ʽ��___________________________________��
��2������1��������Һ����ͨ�����������̼���ټ������ϡ���ᡣ�۲쵽��������_______________________________��
��.������Ʒ��̼��ƵĶ����ⶨ
������ͼ��ʾװ�ã�ͼ�мг�������ȥ������ʵ�飬��ַ�Ӧ�ⶨC�����ɵ�BaCO3������������ȷ��̼��Ƶ�����������
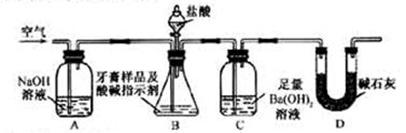
����ʵ����̻ش��������⣺
��3��ʵ����������������ͨ������������ó��˿ɽ���B��C�еķ�Ӧ���⣬���У�_________________________________��
��4��C�з�Ӧ����BaCO3�Ļ�ѧ����ʽ��________________________________��
��5�����и����ʩ�У�������߲ⶨȷ�ȵ���_____________�����ţ���
a.�ڼ�������֮ǰ��Ӧ�ž�װ���ڵ�CO2����
b.�μ�����˹���
c.��A��B֮������ʢ��Ũ�����ϴ��װ��
d.��B��C֮������ʢ�б���̼��������Һ��ϴ��װ��
��6��ʵ����ȷ��ȡ8.00g��Ʒ���ݣ��������βⶨ�����BaCO3ƽ������Ϊ3.94g ������Ʒ��̼��Ƶ���������Ϊ_________��
��7��������Ϊ���زⶨC�����ɵ�BaCO3������ֻҪ�ⶨװ��C������CO2ǰ��������һ������ȷ��̼��Ƶ�����������ʵ��֤�����˷����ⶨ�Ľ������ƫ�ߣ�ԭ����_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij����С���һЩ�������ʺͻ���������ʽ����о���
��1���±�Ϊ�������Ȼ�ͭ��Һ��Ӧ��ʵ�鱨���һ���֣�

����Ӧ����д��ʵ���з�����Ӧ�Ļ�ѧ����ʽ��һ���������ӷ�Ӧ��ֻд���ӷ���ʽ��
�û���Ӧ____________________________________________________________��
���Ϸ�Ӧ____________________________________________________________��
��2����ʯī���缫���������ʵ����������Һ�������������ݡ�
������⣬��������������Һ�л��ɹ۲쵽��������__________________________��__________________________________��
���ʹ���������ӷ���ʽ��________________________��_________________________��
��3����ҵ�Ͽ����������̿���Ҫ�ɷ�ΪMnO2����Ӧ��ұ�������̡�
�����������̿�ұ���̵�ԭ���ǣ��û�ѧ����ʽ��ʾ��
____________________________________________________________________��
��MnO2��H2O2�ֽⷴӦ������������������MnO2�����ữ���H2O2��Һ�У�MnO2�ܽ����Mn2+���÷�Ӧ�����ӷ���ʽ��______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��0.1mol��L-1NaOH��Һ��ͨ�����CO2����Һ�д��ڵ���Ҫ������
A.Na+��CO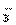 B.Na+��HCO
B.Na+��HCO
C. HCO3-��CO D.Na+��OH-
D.Na+��OH-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ݺ�°��Ƽԭ�����ο��±������ݣ�ʵ�����Ʊ�����Na 2CO3����Ҫ�����ǣ������ƺõı���NaCl��Һ�����ձ��м��ȣ������¶���30��35�棬�����·���������ϸ��NH4HCO3���壬������Ϻ�������30���ӣ����á����˵�NaHCO3���塣����������ˮϴ�ӳ�ȥ���ʣ���ɺ�ת���������У�����2Сʱ���Ƶ�Na2CO3���塣
2CO3����Ҫ�����ǣ������ƺõı���NaCl��Һ�����ձ��м��ȣ������¶���30��35�棬�����·���������ϸ��NH4HCO3���壬������Ϻ�������30���ӣ����á����˵�NaHCO3���塣����������ˮϴ�ӳ�ȥ���ʣ���ɺ�ת���������У�����2Сʱ���Ƶ�Na2CO3���塣
�������ڲ�ͬ�¶��µ��ܽ�ȣ�g/100gˮ����
| 0�� | 10�� | 20�� | 30�� | 40�� | 50�� | 60�� | 100�� | |
| NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | 39.8 |
| NH4HCO3 | 11.9 | 15.8 | 21.0 | 27.0 | ���� | �� | �� | �� |
| NaHCO3 | 6.9 | 8.1 | 9.6 | 11.1 | 12.7 | 14.5 | 16.4 | �� |
| NH4Cl | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.3 | 77.3 |
�٣�35��NH4HCO3���зֽ�
��ش�
��1����Ӧ�¶ȿ�����30��35�棬����Ϊ������35�棬�� ��������30��,�� ��Ϊ���ƴ��¶ȷ�Χ����ȡ�ļ��ȷ���Ϊ ��
��2��������Ϻ�������30���ӣ�Ŀ���� �����ú�ֻ����NaHCO3�����ԭ���� ��������ˮϴ��NaHCO3�����Ŀ���dz�ȥ ���ʣ��Ի�ѧʽ��ʾ����
��3���������õ�ĸҺ�к��� ���Ի�ѧʽ��ʾ��������� ��������һ��������ʹNaCl��Һѭ��ʹ�ã�ͬʱ�ɻ���NH4Cl��
��4�����Դ����Ʒ��NaHCO3�����ķ����ǣ�ȷ��ȡ������ƷW g��������ƿ�м�����ˮ�ܽ⣬��1��2�η�ָ̪ʾ���������ʵ���Ũ��Ϊc(mol/L)��HCl��Һ�ζ�����Һ�ɺ�ɫ����ɫ��ָʾCO32����H����=HCO3����Ӧ���յ㣩������HCl��Һ���ΪV1 mL���ټ�1��2�μ���ָʾ����������HCl��Һ�ζ�����Һ�ɻƱ�ȣ�����HCl��Һ�����ΪV2 mL��
д��������Ʒ��NaHCO3���������ļ���ʽ��NaHCO3��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ������������ԭ��Ӧ���ǣ� ����
A��NH4HCO3 NH3����CO2����H2O
NH3����CO2����H2O
B��Na2O��H2O===2NaOH
C��Na2CO3��H2SO4===Na2SO4��CO2����H2O
D��2H2O 2H2����O2��
2H2����O2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�������ԭ��Ӧ��������ȷ���ǣ� ����
A�����������ڷ�Ӧ��ֻ����ԭ��
B���ǽ��������ڷ�Ӧ��ֻ��������
C������ԭ��ʧ����Խ���仹ԭ��Խǿ
D��Cu2+��Fe2+��������ǿ��Fe��Cu�Ļ�ԭ��ǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݡ��ݴ�����˵����ȷ���ǣ� ��
A�����Լ������ʵ���Ũ��Ϊ9.2 mol��L��1
B��������50 mL��������ͭ��Ӧ�ɵõ���״����SO2 10.3 L
C������200 mL 4.6 mol��L��1��ϡ������ȡ������50 mL
D����������������ˮ���������Һ����������С��49%

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ���ǣ�����
A�������������йص�������������Ӧ�ں�ѹ�����½���ʱ����Ӧ��ЧӦ������
B���������仯���ȵ���ʽ����ʱ������Ӧ��Ϊ���ȷ�Ӧ�����ȷ�Ӧ
C���ⶨ�кͷ�Ӧ�ķ�Ӧ��ʱ��Ϊ���ʵ��ȷ�ԣ�ʹ���Թ����ļ�
D������Դ��̫���ܡ����ܡ����ܡ��������ܵȣ����Ƕ��ǿ�������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com