�����и��������ǰ�һ���������еģ��뽫��Ӧ�������������������ڣ�
��1��N
2H
4��N
2O��NO��HNO
2��N
2O
4��HNO
3��
��Ԫ�صĻ��ϼ���������
��Ԫ�صĻ��ϼ���������
��
��2��CH
4��C
2H
6��C
3H
8��C
4H
10��C
5H
12 ��
���������������1����CH2��ԭ����
���������������1����CH2��ԭ����
��
��3��Ca��Mg��Fe��Cu��Pt ��
���������������
���������������
��
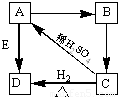
�� A��B��C��D��EΪ�������ʣ���֪A��ҺΪ��ɫ��B��һ�ּC�Ǽ��������D��EΪ�������ʣ��ش�
��1��д��A��B��C�Ļ�ѧʽ��A
CuSO4
CuSO4
��B
Cu��OH��2
Cu��OH��2
��C
CuO
CuO
��D
Cu
Cu
��E
Fe
Fe
��2��д�����и����Ļ�ѧ��Ӧ����ʽ��
A��B��
CuSO4+2NaOH=Cu��OH��2��+Na2SO4
CuSO4+2NaOH=Cu��OH��2��+Na2SO4
��
A��D��
CuSO4+Fe=FeSO4+Cu
CuSO4+Fe=FeSO4+Cu
��
�������������������FeSO
4�����ȷֽ�ʱ�����������ּ��裺
��1����������KClO
3���ȷֽ�ķ�ʽ�ֽ⣬��Ӧ�Ļ�ѧ����ʽΪ
��
��2����������CaCO
3���ȷֽ�ķ�ʽ�ֽ⣬��Ӧ�Ļ�ѧ����ʽΪ
��

 �����и��������ǰ�һ���������еģ��뽫��Ӧ�������������������ڣ�
�����и��������ǰ�һ���������еģ��뽫��Ӧ�������������������ڣ�


 �� I�����и��������ǰ�һ���������еģ��뽫��Ӧ�������������������ڣ�
�� I�����и��������ǰ�һ���������еģ��뽫��Ӧ�������������������ڣ�