
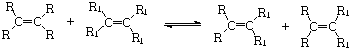
 £®
£®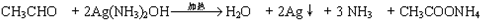 £®
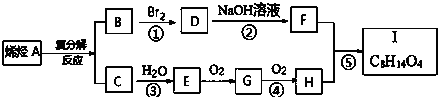
£® ·ÖĪö ŅŃÖŖĶ¬ĪĀĶ¬Ń¹ĻĀ£¬CĻą¶ŌÓŚĒāĘųµÄĆܶČĪŖ14£¬ŌņCµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ28£¬CÄÜÓėĖ®·¢Éś¼Ó³É·“Ӧɜ³ÉE£¬ŌņCĪŖCH2=CH2£¬ĖłŅŌEĪŖCH3CH2OH£¬Ńõ»ÆµĆGĪŖCH3CHO£¬GŌŁ·¢ÉśŃõ»Æ·“Ó¦µĆHĪŖCH3COOH£¬BÓėäå·¢Éś¼Ó³É·“Ó¦µĆDĪŖĀ±“śĢž£¬DŌŚ¼īŠŌĢõ¼žĻĀĖ®½āµĆFĪŖ“¼£¬FÓėH·¢Éśõ„»Æ·“Ó¦µĆI£¬øł¾ŻIµÄ·Ö×ÓŹ½ĪŖC8H14O4£¬æÉĶĘÖŖBĪŖCH3CH=CHCH3£¬DĪŖCH3CHBrCHBrCH3£¬FĪŖCH3CHOHCHOHCH3£¬IĪŖCH3COOCH£ØCH3£©CH£ØCH3£©OOCCH3£¬øł¾ŻĢāÖŠŠÅĻ¢£¬A·¢ÉśĻ©Ģžø“·Ö½ā·“Ó¦µĆBŗĶC£¬ĖłŅŌAĪŖCH3CH=CH2£¬¾Ż“Ė“šĢā£®
½ā“š ½ā£ŗŅŃÖŖĶ¬ĪĀĶ¬Ń¹ĻĀ£¬CĻą¶ŌÓŚĒāĘųµÄĆܶČĪŖ14£¬ŌņCµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ28£¬CÄÜÓėĖ®·¢Éś¼Ó³É·“Ӧɜ³ÉE£¬ŌņCĪŖCH2=CH2£¬ĖłŅŌEĪŖCH3CH2OH£¬Ńõ»ÆµĆGĪŖCH3CHO£¬GŌŁ·¢ÉśŃõ»Æ·“Ó¦µĆHĪŖCH3COOH£¬BÓėäå·¢Éś¼Ó³É·“Ó¦µĆDĪŖĀ±“śĢž£¬DŌŚ¼īŠŌĢõ¼žĻĀĖ®½āµĆFĪŖ“¼£¬FÓėH·¢Éśõ„»Æ·“Ó¦µĆI£¬øł¾ŻIµÄ·Ö×ÓŹ½ĪŖC8H14O4£¬æÉĶĘÖŖBĪŖCH3CH=CHCH3£¬DĪŖCH3CHBrCHBrCH3£¬FĪŖCH3CHOHCHOHCH3£¬IĪŖCH3COOCH£ØCH3£©CH£ØCH3£©OOCCH3£¬øł¾ŻĢāÖŠŠÅĻ¢£¬A·¢ÉśĻ©Ģžø“·Ö½ā·“Ó¦µĆBŗĶC£¬ĖłŅŌAĪŖCH3CH=CH2£¬
£Ø1£©CĪŖCH2=CH2£¬ŅŃÖŖCµÄČ¼ÉÕČČĪŖQ kJ/mol£¬ŌņCĶźČ«Č¼ÉÕµÄČČ»Æѧ·½³ĢŹ½ĪŖCH2=CH2£Øg£©+3O2£Øg£©ØT2CO2£Øg£©+2H2O£Øl£©”÷H=-QkJ/mol£¬
¹Ź“š°øĪŖ£ŗCH2=CH2£Øg£©+3O2£Øg£©ØT2CO2£Øg£©+2H2O£Øl£©”÷H=-QkJ/mol£»
£Ø2£©øł¾ŻÉĻĆęµÄ·ÖĪöæÉÖŖ£¬»ÆŗĻĪļD”¢E”¢G”¢FÖŠ£¬EĪŖCH3CH2OH£¬FĪŖCH3CHOHCHOHCH3£¬¶¼ÓŠōĒ»ł£¬
¹Ź“š°øĪŖ£ŗEF£»
£Ø3£©øł¾ŻÉĻĆęµÄ·ÖĪöæÉÖŖ£¬·“Ó¦¢Ł”¢¢Ś”¢¢Ū”¢¢Ü”¢¢ŻÖŠ£¬ŹōÓŚ¼Ó³É·“Ó¦µÄŹĒ¢Ł¢Ū£¬
¹Ź“š°øĪŖ£ŗ¢Ł¢Ū£»
£Ø4£©øł¾ŻÉĻĆęµÄ·ÖĪöæÉÖŖ£¬AĪŖCH3CH=CH2£¬
¹Ź“š°øĪŖ£ŗCH3CH=CH2£»
£Ø5£©F+H”śI·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ £¬
£¬
¹Ź“š°øĪŖ£ŗ £»
£»
£Ø6£©GĪŖCH3CHO£¬G·¢ÉśŅų¾µ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ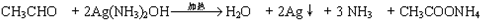 £¬
£¬
¹Ź“š°øĪŖ£ŗ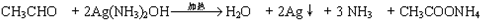 £®
£®
µćĘĄ ±¾Ģāæ¼²éÓŠ»śĪļµÄĶʶĻÓėŗĻ³É£¬ŠčŅŖѧɜ¶Ō·“Ó¦ŠÅĻ¢Ąķ½āŌĖÓĆ£¬½āĢāŹ±×¢ŅāÕĘĪÕ¹ŁÄÜĶŵÄ×Ŗ»Æ£¬ÄѶČÖŠµČ£®


 Ź±æĢ×¼±ø×ÅŹī¼Ł×÷ŅµŌ×ÓÄܳö°ęÉēĻµĮŠ“š°ø
Ź±æĢ×¼±ø×ÅŹī¼Ł×÷ŅµŌ×ÓÄܳö°ęÉēĻµĮŠ“š°ø Źī¼ŁĻĪ½Ó½Ģ²ÄĘŚÄ©Źī¼ŁŌ¤Ļ°Īäŗŗ³ö°ęÉēĻµĮŠ“š°ø
Źī¼ŁĻĪ½Ó½Ģ²ÄĘŚÄ©Źī¼ŁŌ¤Ļ°Īäŗŗ³ö°ęÉēĻµĮŠ“š°ø ¼ŁĘŚ×÷ŅµŹī¼Ł³É³¤ĄÖŌ°ŠĀ½®ĒąÉŁÄź³ö°ęÉēĻµĮŠ“š°ø
¼ŁĘŚ×÷ŅµŹī¼Ł³É³¤ĄÖŌ°ŠĀ½®ĒąÉŁÄź³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÕįĢĒČÜŅŗ | B£® | ĀČ»ÆÄĘČÜŅŗ | C£® | ĒāŃõ»ÆÄĘ | D£® | Ļ”ĮņĖį |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 18O2-½į¹¹Ź¾ŅāĶ¼£ŗ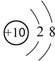 | |
| B£® | Na2O2µÄµē×ÓŹ½£ŗ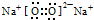 | |
| C£® | HCO3- µÄĖ®½ā·½³ĢŹ½HCO3-+H2O?H2CO3+OH- | |
| D£® | HIµÄµēĄė·½³ĢŹ½ HI?H++I- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Fe3+”¢NH4+”¢SCN-”¢Cl- | B£® | Fe2+”¢H+”¢NO3-”¢SO42- | ||
| C£® | Fe2+”¢Fe3+”¢Na+”¢NO3- | D£® | Fe2+”¢NH4+”¢Cl-”¢OH- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
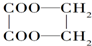 Ź±×ī¼ņ±ćµÄĮ÷³ĢŠč¾ĻĀĮŠÄÄŠ©·“Ó¦£Ø””””£©
Ź±×ī¼ņ±ćµÄĮ÷³ĢŠč¾ĻĀĮŠÄÄŠ©·“Ó¦£Ø””””£©| A£® | ¢Ł¢Ś¢Ū¢Ü¢Ž | B£® | ¢Ż¢Ś¢Ł¢Ū¢Ž | C£® | ¢Ž¢Ū¢Ł¢Ś¢Ż | D£® | ¢Ł¢Ś¢Ż¢Ū¢Ž |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŹÆÓĶÖ÷ŅŖŗ¬C H Į½ÖÖŌŖĖŲ | |
| B£® | ŹÆÓĶŹĒÖ÷ŅŖÓÉø÷ÖÖĶéĢž »·ĶéĢžŗĶ·¼ĻćĢžĖł×é³ÉµÄ»ģŗĻĪļ | |
| C£® | ŹÆÓĶÓŠ¹Ģ¶ØµÄ·Šµć£¬¹ŹæÉ·ÖĮó | |
| D£® | ŹÆÓĶ·ÖĮóµĆµ½µÄĘūÓĶŹĒ»ģŗĻĪļ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ±ź×¼×“æöĻĀ£¬22.4LøżĶéĖłŗ¬ÓŠµÄ·Ö×ÓŹżĪŖNA | |
| B£® | 1mol¼×»ł£Ø-CH3£©Ėłŗ¬µē×ÓŹżĪŖ9NA | |
| C£® | ±ź×¼×“æöĻĀ£¬CH4ŗĶC2H4µÄ»ģŗĻĘųĢå22.4L£¬Ėłŗ¬µÄ·Ö×ÓŹżĪŖŌ¼ĪŖNA | |
| D£® | 26g C2H2ŗĶ±½ÕōĘųµÄ»ģŗĻĘųĢåÖŠĖłŗ¬µÄCŌ×ÓŹżĪŖ2NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | µĪ¼ÓŹÆČļŹŌŅŗĻŌŗģÉ«µÄČÜŅŗ£ŗFe3+”¢NH4+”¢Cl-”¢SCN- | |
| B£® | pHĪŖ1µÄČÜŅŗ£ŗFe2+”¢K+”¢ClO-”¢Cl- | |
| C£® | c£ØH+£©=10-12 mol•L-1µÄČÜŅŗ£ŗK+”¢Ba2+”¢Cl-”¢NO3- | |
| D£® | “ęŌŚ½Ļ¶ąµÄH+”¢SO42-”¢NO3-µÄČÜŅŗ£ŗAl3+”¢CH3COO-”¢Cl- |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com