
| |||||||||||||||||||||||
(1) |
������ƽ���ձ���200mL����ƿ����ͷ�ιܣ������������ |
(2) |
5SO2��2MnO4����2H2O��5SO42����2Mn2+��4H+ |
(3) |
|
(4) |
��ֹ������������������ܻ�������Ը��������Һ�У�ʹ����������,����ǰ�����������������ʢ����������������(��װ���ñ�ű�ʾ�ش𣬻�����������������ʢ��������������������ֵ) |
(5) |
ͨ���������ʹ��죬��������δ�����Ը��������Һ��ַ�Ӧ���Ѿ����ų� |


 �ݾ�ѵ������ϵ�д�
�ݾ�ѵ������ϵ�д� С����ȫ�ܼ��ϵ�д�
С����ȫ�ܼ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

 ��
�� ��ʾ�ܱ�������
��ʾ�ܱ�������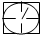 ��ʾ�������ٹܣ���λʱ����ͨ�����������㶨���������������������ã���
��ʾ�������ٹܣ���λʱ����ͨ�����������㶨���������������������ã��� ��ʾ���������������������տ�����Ŀ���������ʾ���������
��ʾ���������������������տ�����Ŀ���������ʾ���������| 3.2 |
| at |
| 3.2 |
| at |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

 ��
�� ��ʾ�ܱ�������
��ʾ�ܱ�������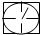 ��ʾ�������ٹܣ���λʱ����ͨ�����������㶨���������������������ã���
��ʾ�������ٹܣ���λʱ����ͨ�����������㶨���������������������ã��� ��ʾ���������������������տ�����Ŀ���������ʾ���������
��ʾ���������������������տ�����Ŀ���������ʾ����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������



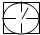
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2007-2008ѧ������ʡ�������������и������ϣ���ѧʵ���ۺϼ���Ծ��������棩 ���ͣ������

 ��
�� ��ʾ�ܱ�������
��ʾ�ܱ������� ��ʾ�������ٹܣ���λʱ����ͨ�����������㶨���������������������ã���
��ʾ�������ٹܣ���λʱ����ͨ�����������㶨���������������������ã��� ��ʾ���������������������տ�����Ŀ���������ʾ���������
��ʾ���������������������տ�����Ŀ���������ʾ����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������̨��2001��6��5����ÿ�춼Ҫ����47���ص���еĿ�������Ԥ��������SO2�Ϳ���������ĺ�����Ŀǰ�ⶨ������SO2������Ҫ��������ԭ��Ӧ������������������ٹܣ���λʱ����ͨ�����������㶨��������������������ã����衱���ܱ������������������������ȡ������ܡ�-�� ��0.0001mol?L-1�����Ը��������Һ��������������Ʒ����Һ��pH��ֽ��������ҩƷ�������һ��ʵ��װ�ã��ⶨ�����ڵ���������SO2�Ϳ���������ĺ�������֪5SO2+2H2O+2MnO4-����ɫ��=5SO42-+2Mn2+����ɫ��+4H+�������������������£�
��0.0001mol?L-1�����Ը��������Һ��������������Ʒ����Һ��pH��ֽ��������ҩƷ�������һ��ʵ��װ�ã��ⶨ�����ڵ���������SO2�Ϳ���������ĺ�������֪5SO2+2H2O+2MnO4-����ɫ��=5SO42-+2Mn2+����ɫ��+4H+�������������������£�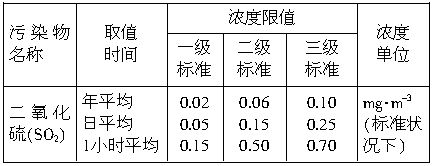
��1�������ⶨ������SO2�Ϳ���������ĺ�����ʵ��װ��ͼ����ָ�������е�ҩƷ����
��2�����������ٹ�����������Ϊ5000cm3?min-1����140minʱ20mL0.0001mol?L-1�����Ը��������Һǡ����ɫ��������ҹ������������Ͳⶨ����ж����ⶨ�ص�Ŀ�����SO2�ĺ�������_________�������֣���������������
��3����Ҫ�ⶨ�����п���������ĺ�������Ҫ�����������__________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com