
�������һ����Ҫ�ķǽ������ϣ��Ʊ��������Ҫ�������£�
�ٸ�������̼��ԭ���������Ƶôֹ�
�ڴֹ������HCl���巴Ӧ�Ƶ�SiHCl3��Si��3HCl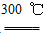 SiHCl3��H2
SiHCl3��H2
��SiHCl3�����H2��1 000��1 100 �淴Ӧ�Ƶô��衣
��֪SiHCl3����H2Oǿ�ҷ�Ӧ���ڿ���������ȼ��
��ش��������⣺
(1)�ڢٲ��Ʊ��ֹ�Ļ�ѧ��Ӧ����ʽΪ________________________________��
(2)�ֹ���HCl��Ӧ��ȫ�������õ���SiHCl3(�е㣭33 ��)�к�������SiCl4(�е�57.6 ��)��HCl(�е㣭84.7 ��)���ᴿSiHCl3���õķ���Ϊ________��
(3)��SiHCl3�����H2��Ӧ�Ʊ������װ������ͼ(��Դ���г�װ����ȥ)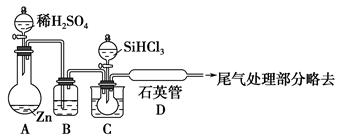
��װ��B�е��Լ���________��װ��C�е���ƿ��Ҫ���ȣ���Ŀ����___________________________________________________________��
�ڷ�Ӧһ��ʱ���װ��D�й۲쵽��������________��װ��D���ܲ�����ͨ�����ܵ�ԭ����__________________________________________________��
װ��D�з�����Ӧ�Ļ�ѧ����ʽΪ____________________________________��
��Ϊ��֤�Ʊ�����ʵ��ijɹ��������Ĺؼ��Ǽ��ʵ��װ�õ������ԣ����ƺ÷�Ӧ�¶��Լ�________________________________________________��
��Ϊ������Ʒ�����Ƿ��������ʣ���������ϡ�����ܽ⣬ȡ�ϲ���Һ�����ټ�����Լ���(��д��ĸ����)________��
a����ˮ��b����ˮ��c��NaOH��Һ��d��KSCN��Һ e��Na2SO3��Һ

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Na2S2O3��5H2O���׳ƺ�������մ�������ҵ���õ�һ�ֶ�Ӱ����
��һ)��������;���Ʊ�������
��֪��Ӧ��4FeS+7O2  2Fe2O3+4SO2��Na2SO3+S
2Fe2O3+4SO2��Na2SO3+S Na2S2O3
Na2S2O3
��1������ԭ��FeS�ڷ�Ӧ(a)��(b)�е����۷���ȣ�_________��
��2������88gFeS����NaOH��Һ����SO2��������Ϊ96%��������Ʊ���������Ϊ____�ˣ���ȷ��0��1�ˣ�
��������ҵ���Ƶõĺ��������п��ܺ����������������ƺ����������ʡ�Ϊ�˲ⶨij������Ʒ�ijɷ֣���ȡ����������ͬ�ĸ���Ʒ���ֱ������ͬŨ�ȵ�������Һ30mL����ַ�Ӧ����˳�������Һʹ���ɵ�SO2ȫ���ݳ���Na2S2O3+ H2SO4® Na2SO4+ SO2��+ S��+ H2O��������й�ʵ���������£���״������
| | ��һ�� | �ڶ��� | ������ |
| ��Ʒ������/g | 7��54 | 15��08 | 35��00 |
| ������������/L | 0��672 | 1��344 | 2��688 |
| �������/g | 0��80 | 1��60 | 3��20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��֪AgNO3������ȷֽ��������ֵ��ʺͺ���ɫ���塣������ijЩװ�ô��Բⶨ�������������ֽ�Ҳ�����뷴Ӧ�����ʵ��������Ĵ��ȣ��������й�ʵ��(װ���б�Ҫ������̨�����С��ƾ��Ƶ�����ȥ)����д���пհס�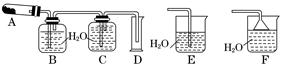
��1��д��AgNO3���ȷֽ�Ļ�ѧ����ʽ��_______________________________________________________________��
��2���ⶨAgNO3�Ĵ��ȣ���ѡ����A��B��C��D��ɵ�װ�ã������в���������____________���ô�������ĺ����____________________________________________��
��3��Bƿ�еĿ�����ʵ����________(��С����ޡ�)Ӱ�죬������____________________________________________________________________________��
��4������Ľ�װ�ú�ȡ����������4.00 g����A�л������ȣ�����Ӧ��ȫ����������ͨ��B��Cװ�ú����Ͳ��ˮ�������������ɱ�״������������Ϊ112 mL���������Ĵ���Ϊ________��
��5���������Cu(NO3)2��������ͭ������ȷֽ���������Ӧͨ��װ��________(�E����F��)����������__________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ҵ�ϳ�����������ʢװ��Ũ���ᡣΪ�о����ʲ�������Ũ����ķ�Ӧ��ijѧϰС�����������̽�����
��̽��һ��
��1������ȥ�������������������̼�ظ֣�������Ũ�����У�10���Ӻ���������ͭ��Һ�У�Ƭ�̺�ȡ���۲죬�������������Ա仯����ԭ����__________________��
��2����ȡ����6.0 g����15.0 mLŨ�����У����ȣ���ַ�Ӧ���ռ�������Y��
��ͬѧȡ336 mL����״��������Yͨ��������ˮ�У�������Ӧ��SO2+Br2+2H2O=2HBr+H2SO4
Ȼ���������BaCl2��Һ�����ʵ�������ø������2.33 g���ɴ���֪����Y��SO2���������Ϊ______��
��̽������
��������ʵ����SO2��������Ľ������ͬѧ��Ϊ����Y�л����ܺ���H2��Q���塣Ϊ�����������̽��ʵ��װ�ã�ͼ�мг�����ʡ�ԣ���
��3��װ��B���Լ���������_________________________________________��
��4����Ϊ����Y�л�����Q��������______________________________�����û�ѧ����ʽ��ʾ����
��5��Ϊȷ��Q�Ĵ��ڣ�����װ��������M��______��ѡ����ţ���
a.A֮ǰ b.A��B�� c.B��C�� d.C��D��
��6���������Y�к���H2��Ԥ��ʵ������Ӧ��____________________________��
��7����Ҫ�ⶨ���������Y��H2�ĺ�������״����Լ��28 mL H2���������ò���H2����ķ����⣬�ɷ�ѡ�����������ķ����������жϲ�˵������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ��ȤС���ͬѧ������ͼ��ʾʵ��װ�ý���ijЩ������Ʊ������ʵ�ʵ��(ͼ�мг�װ����ʡ��)���밴Ҫ����գ�
��.̽�������백���ķ�Ӧ
(1)Ϊ��ȡ���ﰱ�����ɽ�װ��C��________(��װ�ñ��)���ӣ�װ��C�е���ƿ�ڹ�����ѡ��________��
a����ʯ�� b���Ȼ��� c������������ d����ʯ��
(2)װ��A��E��E���ӿ���ȡ�����������������������Eװ���ڵ�ҩƷ������________________��
(3)װ��F������̽�������백��(��֪�����백���ɷ�����Ӧ��3Cl2��2NH3===N2��6HCl)�ķ�Ӧ��ʵ��ʱ���ɼ�1��3���ر�2��������ƿ��ͨ��________��Ȼ��ر�1��3����2������ƿ�л���ͨ��һ��������һ�����塣ʵ��һ��ʱ�����ƿ�ڳ���Ũ��İ��̲��������ڱ����ᣬ�����һ��ʵ�鷽�������ù����е�������______________________________________________________________________________��
��.̽��ijЩ���ʵ�����
(4)����װ��A��E�������ʵ��Ƚ�Cl����Br���Ļ�ԭ��ǿ������֤�����۵�ʵ��������______________________________________________________��
(5)������װ��A��E������ϩ����ˮ��Ӧ��ʵ�飬д����Ӧ�Ļ�ѧ����ʽ________________________________________________________________��
(6)��װ��B��C�ֱ���F��������H2S��SO2��Ӧ��ʵ�顣F����ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ______________________��F���ձ������������________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ��ȤС��Ϊ̽��SO2�����ʣ�����ͼ��ʾװ�ý���ʵ�顣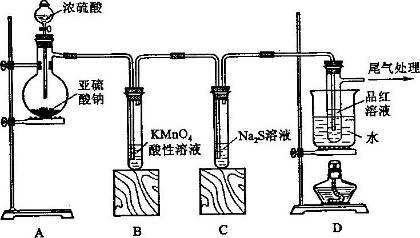
��ش��������⣺
��1��װ��A��ʢ���������Ƶ�����������_________�����з�����Ӧ�Ļ�ѧ����ʽ_______________________��
��2��ʵ������У�װ��B��C�з���������ֱ���____________��___________��װ��B�з�����Ӧ�����ӷ���ʽΪ_________________________________��
��3��װ��D��Ŀ����̽��SO2��Ʒ�����õĿ����ԣ���д��ʵ�����������________________________��
��4��β���ɲ���___________________��Һ���ա�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������ش�ijͬѧ��̽��Ũ���ᡢϡ���ᡢŨ���ᡢϡ����ֱ���ͭ��Ӧ��ʵ���з��ֵ��й����⡣
��.̽����������������������ǿ��������ͭ��Ӧ�Ļ�ԭ���������
(1)�ֱ���ʢ�е���ͭƬ����֧�Թ��м���������Ũ���ᡢϡ���ᡢŨ���ᡢϡ����,ʵ������¼���±�:
| | �� | ʵ���� |
| a | Ũ���� | ���Ⱥ�����Ӧ,������ɫ�̼������� |
| b | ϡ���� | ����Ҳ��������Ӧ |
| c | Ũ���� | �����ȼ�������Ӧ,��������ɫ���� |
| d | ϡ���� | �ȷ�����Ӧ,������ɫ���� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ҵ�ϳ�����������ʢװ��Ũ���ᡣij��ȤС���ͬѧ���֣���һ������������Ũ�������ʱ���۲쵽������ȫ�ܽ⣬�������������塣ʵ�������������Լ��� 0.01 mol/L ����KMnO4��Һ��0.10 mol/L KI��Һ��������ˮ��������Һ������ˮ������Э������̽��������Һ������ijɷ֡�
��������롿
��������Һ�еĽ������ӿ��ܺ���Fe2����Fe3���е�һ�ֻ����֣�
�����������п϶����� ���塣
��ʵ��̽����
| | ʵ����� | Ԥ������ | �� �� |
| ��֤����� | ����٣�ȡ����0.01 mol/L ����KMnO4��Һ������������Һ�� | | |
| ����ڣ� | | ����Fe3�� | |
| ��֤����� | ����������ͨ������װ�� | | �������ֻ��������� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�� (1)ij��ѧ��ȤС����������װ����ѡȡ��Ҫ��װ����ȡ (NH4)2SO4��Һ�����ӵ�˳���ýӿ������ĸ��ʾ���ǣ�a 
(2)��װ��C������Һ����뿪�IJ���������__ _______��
װ��D�������� ��
��4�֣�Ϊ����Ȼ�淋ľ��ü�ֵ���ҹ���ѧ�����������������þ�ȷֽ��Ȼ���ư������õ���ʽ�Ȼ�þ(MgOHCl)�Ĺ��ա�ijͬѧ���ݸ�ԭ����Ƶ�ʵ��װ����ͼ��
��ش��������⣺
(1) װ��A�з�����Ӧ���ɼ�ʽ�Ȼ�þ�Ļ�ѧ����ʽΪ_________ ____
װ��B�м�ʯ�ҵ�������_____ __
(2) ��Ӧ�����г���ͨ��N2�����������㣺һ��ʹ��Ӧ�����İ�����ȫ��������ϡ���������գ�����_______________ ______
(3) װ��C���Թ��з�Ӧ�����ӷ���ʽΪ ______________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com