
���� ��1���¶Ƚϸ�ʱ��˫��ˮ�ֽ⣬��ȩ�ӷ���
��2���ؽ��������������ӷ�Ӧ�������ܵ����
��3�������ˮ���Լ��ԣ���ҺΪ��������������ˮ�⣻
��4���ٸ��ݵζ���������ѡ��ʹ�õ�������
�ڸ��ݵζ�����ǰ��ҺΪΪ��ɫ���ζ�����ʱ���������Һ��Һ�������н��
��HCOOH��Ӧ��C��+2������Ϊ������̼��+4�ۣ�MnO4 -��Mn��+7�۽�ΪMn2+�е�+2�ۣ�����������ԭ��Ӧ��ʧ�����غ���ԭ�Ӹ����غ�ֱ���д����ʽ����������ѧ����ʽ���м��㣮
��� �⣺��1���������������ֽ⣺2H2O2$\frac{\underline{\;\;��\;\;}}{\;}$2H2O+O2�����¶Ƚϸ�ʱ��˫��ˮ�ֽ⣬��ȩ�е�ֻ��-19.5�棬�ӷ�����Ӧ�¶���ÿ�����30-70��֮�䣬�¶Ȳ����ߣ��ܷ�ֹH2O2�ֽ�ͼ�ȩ�ӷ���
�ʴ�Ϊ����ֹH2O2�ֽ�ͼ�ȩ�ӷ���
��2���Ʊ�ʱ�ڻ����Һ��Ҫ���������������Ƽ�ȩ��������Ӧ�⣬��Ҫ����������Na2S��Һ���������ؽ������ӽ�������������������ˮ�����Գ�ȥ�ؽ������ӣ�
�ʴ�Ϊ����ȥ�ؽ������ӣ�ʹ�ؽ��������γ����������ȥ����
��3�����������Ϊ��������ӣ�������ˮ���������ԣ�������ҺpH 7��8����ҺΪ�����������Ƽ����ˮ�⣬�������������Һ��pH 7��8���������ԣ��ܳ�ȥ���ᣬ
�ʴ�Ϊ����ֹ�����ˮ�⣨���ȥ���ᣩ��
��4���ٵζ���������Ҫʹ�õζ��ܺ���ƿ����B��D��ȷ��
�ʴ�Ϊ��B��D��
�ڵζ�����֮ǰ��ҺΪ��ɫ���ζ�����ʱ���������Һ��������Һ��Ϊ��ɫ�����Եζ��յ���ɫ�仯Ϊ����ɫ��Ϊ��ɫ����������Һ����ɫ��
�ʴ�Ϊ����ɫ��Ϊ��ɫ����������Һ����ɫ��
��HCOOH��Ӧ��C��+2������Ϊ������̼��+4�ۣ�MnO4 -��Mn��+7�۽�ΪMn2+�е�+2�ۣ�Ҫʹ�������뻹ԭ����ʧ���������H2C2O4ϵ��Ϊ5��MnO4 -ϵ��Ϊ2�����ԭ�Ӹ����غ㣬��Ӧ����ʽ��2MnO4-+5HCOOH+6H+=2Mn2++5CO2��+8H2O��H2C2O4��Ӧ��C��+3������Ϊ������̼��+4�ۣ�MnO4 -��Mn��+7�۽�ΪMn2+�е�+2�ۣ�Ҫʹ�������뻹ԭ����ʧ���������H2C2O4ϵ��Ϊ5��MnO4 -ϵ��Ϊ2�����ԭ�Ӹ����غ㣬��Ӧ����ʽ��5H2C2O4+2MnO4-+6H+=2Mn2++10CO2��+8H2O����$\frac{5}{2}$Ca��HCOO��2��5CaC2O4��2KMnO4����ԭ�������Ca��HCOO��2��CaC2O4�����ʵ����ֱ�Ϊx��y����130x+128y=0.388��$\frac{4}{5}$x+$\frac{2}{5}$y=0.1��20��10-3����ã�x=0.002mol��y=0.001mol��ԭ�������Ca��HCOO��2��CaC2O4�����ʵ���֮��Ϊ0.002mol��0.001mol=2��1��
�ʴ�Ϊ��2��1��
���� ���⿼�������Ʊ����漰ʵ�����ԭ�������衢�����ⶨ���й����⣬�������ʵ��Ʊ��������ʡ�ʵ�鷽�����ԭ�����ζ�ԭ�����ǽ��ؼ�����Ŀ�Ѷ��еȣ��ۺ�������ѧ����ʵ��̽��������


 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ������Ϊ���� | �����Լ� | ���Ӳ��� |
| A | Fe��Al���� | NaOH��Һ | �ڹ�����NaOH��Һ�г�ַ�Ӧ����� |
| B | Na2O2��Na2O������ | O2 | �ڴ������м��� |
| C | FeCl2��FeCl3����Һ | Fe�� | �������Fe�ۣ���ַ�Ӧ����� |
| D | Na2CO3��NaHCO3�� ��Һ | CO2 | ͨ�������CO2 |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ͭ˿����������Һ��Ӧ��Cu+Ag+�TCu2++Ag | |
| B�� | ����̼���������������Ʒ�Ӧ��HCO3-+OH-+Ca2+�TCaCO3��+H2O | |
| C�� | ϡ����������������Һ��ϣ�H++OH-�TH2O | |
| D�� | ����ˮ��Ӧ��2Na+2H2O�T2Na++2OH-+H2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ҵ��ͨ���������״̬MgCl2��ȡ����þ | |
| B�� | �ڵڢڡ��ܲ����У���Ԫ�ؾ������� | |
| C�� | �ó����ʯ��ˮ�ɼ���NaHCO3��Na2CO3 | |
| D�� | ��ȡNaHCO3�ķ�Ӧ���������ܽ��С��NaCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ʱ��/s | 0 | 500 | 100 0 | 150 0 |
| c��N2O5��/mol•L-1 | 5.00 | 3.52 | 2.50 | 2.50 |
| A�� | 500s��N2O5�ֽ�����Ϊ2.96��10-3mol•L-1•s-1 | |
| B�� | T1�¶��µ�ƽ�ⳣ��ΪK1=125��ƽ��ʱN2O5��ת����Ϊ50% | |
| C�� | ��ƽ��������������䣬�����������ѹ����ԭ����1/2����ƽ��ʱc��N2O5����5.00mol•L-1 | |
| D�� | T1�¶��µ�ƽ�ⳣ��ΪK1��T2�¶��µ�ƽ�ⳣ��ΪK2����T1��T2����K1��K2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 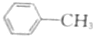 | B�� | CH2�TCHCH2OH | C�� | 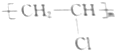 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��˪���Ǽ��������� | B�� | Na3As04�ǿ����Ե����� | ||
| C�� | ��˪�о綾�����л�ԭ�� | D�� | AsH3���⻯��ȶ��Խ�ǿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | c��HF����c��F-�� | B�� | c��Na+����c��F-�� | ||
| C�� | c��F-��-c��HF��=c��H+��-c��OH-�� | D�� | c��HF��+c��F-��=0.1mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ֻ��Na2O | B�� | ֻ��Na2O2 | C�� | Na2O2��Na2O | D�� | ��ȷ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com