
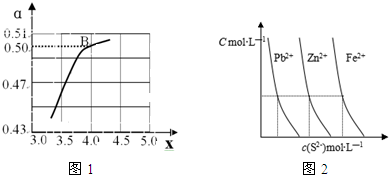
���� ��1��������ƽ�ⳣ�����¶ȱ仯�����жϷ�Ӧ���ȷ��ȣ�
������Ӧ��Ũ�ȣ�ƽ��������У�����x�����ͼ�����ݷ�����ϻ�ѧ����ʽ���㰱��ת���ʣ�
�ۺ��º��������£�����ϡ�����壬��Ӧ��ϵ�и���ֵ�Ũ�Ȳ��䣬ƽ�ⲻ�ƶ���
��1����ˮ�������ɵ�c��H+��=c��OH-����Kw=c��H+����c��OH-�����ȼ����������c��OH-�����ٽ��Kw����c��H+�����ó���Ӧ�����Һ��pH��
��2������CH3COOH?H++CH3COO-����ƽ�⣬������Ũ�ȼ�С��pH���û����Һ��c ��Na+����c ��CH3COO-������ˮ��С�ڵ��룻
��1������ͼ2�жϳ�PbS��s����ZnS��s����FeS��s�����ܶȻ����ܽ�ȴ�С���ܽ��Խ������������ܽ⣻
��2������PbS��s����ZnS��s����FeS��s�����ܽ�ȴ�С���������ܽ�ƽ���жϳ���ת�������
��� �⣺��ƽ�ⳣ�����¶�����С��˵������ӦΪ���ȷ�Ӧ����H��0���ʴ�Ϊ������
��2NH3 ��g��+CO2 ��g��?CO��NH2��2 ��l��+H2O ��l����ͼ������жϣ�������Ũ��ƽ��������У�
B�㴦x=4��ԭ�����е�NH3��CO2�����ʵ���֮��Ϊ4��������̼ת����Ϊ0.50�����谱��Ϊ4mol��������̼Ϊ1mol����Ӧ�Ķ�����̼Ϊ0.50mol��
���ݻ�ѧ����ʽ2NH3��g��+CO2��g��?CO��NH2��2��l��+H2O��l����Ӧ���ĵİ���Ϊ1mol��
NH3��ƽ��ת����=$\frac{1}{4}$��100%=25.0%��
�ʴ�Ϊ��c��NH3������ƽ�������ƶ���CO2ת��������25.0��
�۴ﵽƽ��ʱ���ں��º��������³���ϡ�����壬���ڸ���ֵ�Ũ��û�б仯����ѧƽ�ⲻ���ƶ���CO��NH2��2��l�����������䣬
�ʴ�Ϊ�����䣻
��1����ˮ�������ɵ�c��H+��=c��OH-����Kw=c��H+����c��OH-��=4��10-14��������c��OH-��=$\frac{0.9V-0.1V}{2V}$=0.4mol/L��
�ɸ��¶��µ�Kw��֪��c��H+��=$\frac{4��1{0}^{-14}}{0.4}$mol/L=10-13mol•L-1������pH=13��
�ʴ�Ϊ��13��
��2������CH3COOH?H++CH3COO-����ƽ�⣬����CH3COONa���壬c ��CH3COO-��������ƽ�������ƶ������ƴ�����룬��Һ������Ũ�ȼ�С��pH����
�û����Һ��c ��Na+����c ��CH3COO-�������������ӵ�ˮ��̶�С�������̶ȣ�����c��CH3COOH����c ��CH3COO-����
�ʴ�Ϊ������
��1������ͼ2��֪��PbS��s����ZnS��s����FeS��s�����ܶȻ���С��ϵΪ��Ksp��CuS����Ksp��ZnS����Ksp��FeS�������ܽ��PbS��ZnS��FeS����������ֳ����м����ᣬ�����ܽ�����ܽ������FeS��
�ʴ�Ϊ��FeS��
��2���������ɵ�ZnS��Һ�е�����������ͬŨ�ȵ�Cu2+��Fe2+����Һ������Ksp��PbS����Ksp��ZnS����Ksp��FeS�����ܽ��PbS��ZnS��FeS������ZnS������ת��Ϊ�����ܵ�PbS��
�ʴ�Ϊ��PbS��
���� ���⿼���˻�ѧƽ��Ӱ�����ء�����϶����жϼ���Һ���������ҺpH�ļ��㡢�������ܽ�ƽ�⼰����ת����֪ʶ����Ŀ�ѶȽϴ����������ϴ����Ϊ�ۺϣ�ע����ȷˮ�ĵ��롢������ʵĵ��롢����ˮ�⡢�������ܽ�ƽ���֪ʶ���ɽ��ע���Ϻ���Һ�е������ж�Ϊ���Ĺؼ���������������ѧ���ķ������������������Ӧ����ѧ֪ʶ���ʵ�������������


 ���ĺ����Ͼ�������ϵ�д�
���ĺ����Ͼ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʯ��ׯ������ѧ�߶��Ͻο�һ��ѧ���������棩 ���ͣ�ѡ����
����˵����ȷ���ǣ� ��
A������FeCl3��Һʱ����FeCl3�����������������У�Ȼ������ˮϡ�͵������Ũ��
B��Ϊ�����к͵ζ����ߣ��ڵζ������У�ÿ����ͬʱ����pH�Ʋⶨһ����ƿ����Һ��pH
C���ⶨ��Һ��pHʱ��Ӧ�Ƚ�pH��ֽ��ʪ��Ȼ����м��
D��Ϊȷ�ⶨ������NaOH��Һ��Ӧ���к��ȣ�������ͼ�����ʵ���Ӧ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�켪��ʡ�����и�����ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
O2F2���Է�����Ӧ��H2S+4O2F2=SF6+2HF+4O2������˵����ȷ����
A����������������
B��O2F2�������������ǻ�ԭ��
C��������4.48LHF����ת��0.8mol����
D����ԭ���������������ʵ���֮��Ϊ1��4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

 ��D��E�ķ�Ӧ����ȡ����Ӧ��
��D��E�ķ�Ӧ����ȡ����Ӧ��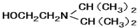 ��
��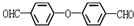 ��
�� ���ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�CH3CH2Br$��_{��}^{NaOH��Һ}$CH3CH2OH$��_{Ũ���ᣬ��}^{CH_{3}COOH}$CH3COOCH2CH3��
���ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�CH3CH2Br$��_{��}^{NaOH��Һ}$CH3CH2OH$��_{Ũ���ᣬ��}^{CH_{3}COOH}$CH3COOCH2CH3���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
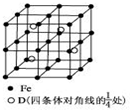 A��B��C��D�ֱ�������ֲ�ͬ�Ķ�����Ԫ�أ�A��BԪ��ԭ�ӵļ۵����Ų��ֱ�Ϊns1��ns2np2��CԪ�ص�����������������Ӳ�����3����DԪ��ԭ�ӵ�M���Ӳ��p�ܼ�����1�����ӣ�
A��B��C��D�ֱ�������ֲ�ͬ�Ķ�����Ԫ�أ�A��BԪ��ԭ�ӵļ۵����Ų��ֱ�Ϊns1��ns2np2��CԪ�ص�����������������Ӳ�����3����DԪ��ԭ�ӵ�M���Ӳ��p�ܼ�����1�����ӣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ԫ��ԭ�ӵ���������������Ԫ�ص�����ϼ� | |
| B�� | �����ԭ���У�����ԭ�Ӻ˽Ͻ����������˶��ĵ��������ϸ� | |
| C�� | P��S��Cl�ǽ����Ժ�����������Ӧ��ˮ��������Ծ�������ǿ | |
| D�� | Ԫ�����ڱ���λ�ڽ����ͷǽ����ֽ��߸�����Ԫ�����ڹ���Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3COOH��HCN��Ϊ���ᣬ���ԣ�HCN��CH3COOH | |
| B�� | 25��ʱ��ˮ���������c��H+����Ϊ 10-9mol/L�������ᣬ���Ũ�ȣ�HCN��CH3COOH | |
| C�� | 25��ʱ��Ũ�Ⱦ�Ϊ0.1mol/L ��CH3COONa��NaCN��Һ�У�pH��С��CH3COONa��NaCN | |
| D�� | 25��ʱ����ȡ100m LpH=3��CH3COOH��Һ��ˮϡ�͵�1000m L��������Һ��ˮ�����c��OH-����10-10mol/L�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ۢ� | B�� | �ڢۢ� | C�� | �ڢܢ� | D�� | �٢ܢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com