
��ͬ������������Һ����ȫ����ʱ����Һ��pH��ͬ��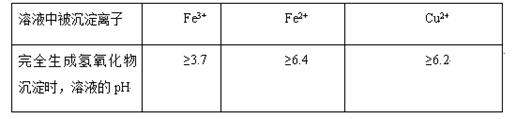
�Ȼ�ͭ���壨CuCl2��2H2O���к�FeCl2���ʣ�Ϊ�Ƶô����Ȼ�ͭ���壬���Ƚ����Ƴ�ˮ��Һ��Ȼ��������ʾ�IJ�����������ᴿ��
��1���������������ʺ���������X���� ������ţ���
| A��NaClO | B��H2O2 | C��KMnO4 | D��Cl2 |
��1��B��D����2��CuO��Cu(OH)2��CuCO3����3��2Fe3++6H2O+3CuO=2Fe(OH)3��+3Cu2+��
��4�����ܣ���CuCl2��Һ������ˮ�Ȼ��������м��ȡ���5��5
���������������1�����Ȼ�ͭ���壨CuCl2��2H2O���к�FeCl2���ʣ�Ϊ�Ƶô����Ȼ�ͭ����Ӧ�ð����ʳ�ȥ������Fe2+�γɳ�����pH��Cu2+�γɳ�����pH������������׳�ȥ����������������Fe2+����ΪFe3+�ͺ����׳�ȥ�ˡ���ѡ��������ʱ��Ҫ�����µ��������ӡ�����Ӧ��ѡ����ɫ������B��H2O2��D��Cl2��(2)��������Y���Ե�����Һ��pHֵ�����Ҳ��������ʡ����Լ���CuO��Cu(OH)2��CuCO3��Cu2(OH)2CO3�ȡ���3��������CuOΪ������Ӧ�����ַ���ʽΪ2Fe3++6H2O+3CuO=2Fe(OH)3��+3Cu2+��(4) CuCl2�����ӷ���ǿ�����������Ӧ�õ����Σ���ֱ�Ӽ���������Һ��ˮ�������HCl������ˮ�ֵ���������������õ���ΪCu(OH)2,���������ֱ�������ᾧ�õ�CuCl2��2H2O���塣Ϊ��ֹ��ˮ��±��ʣ�Ӧ�ý���Һ������ˮ�Ȼ��������м��ȾͿ���CuCl2��2H2O���塣��5������������ͭ��������Ksp��c��Cu2������ c2��OH������2��10��20mol2��L��2����c(Cu2��)=0��02mol/L,��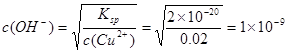 c��H+��=Kw��c(OH-)=10-14��10-9=10-5;����pH=5��
c��H+��=Kw��c(OH-)=10-14��10-9=10-5;����pH=5��
���㣺�����Լ���ѡ�����ʵij�ȥ��������Һ����Եķ������ᾧ�ķ�����ʵ�鷽������Ƶ�֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��12�֣�ijС��������ȷ�Ӧʵ�飬װ����ͼ��ʾ��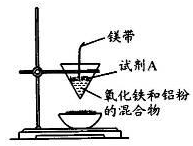
��1�����ȷ�Ӧԭ��____��д��ѧ����ʽ�����Լ�AΪ____��д��ѧʽ����ʵ������____���к�ɫ�������ɡ�
��2���Ժ�ɫ�������ʵ���ɣ�С�����������̽��
����I�ú�ɫ����Ϊ��
�����ú�ɫ����Ϊ���������������Ļ����
����Ʒ�����ʵ�顿
| ʵ �� �� �� | �� �� | �� �� |
| ��ȡ������ɫ�������Թ��м�ϡ���� | ����ȫ���ܽ⣬�����ݲ��� | ����I������������� |
| �ڵ��뼸��0.01mol/LKSCN��Һ | ��Һ����ɫ |
| ʵ���� | ʵ�鲽�� |
| 1 | �ٵμӱ���FeCl3��Һ4�Σ������ |
| �ڵμӼ���NaOH��Һ | |
| | �ٵμ�4��1mol/LKSCN��Һ |
| �ڵμӼ���NaOH��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��12�֣�ij�о���ѧϰС���������Ѽ�����Ϣ���ء��ơ��ơ�þ�Ȼ��ý�������CO2������ȼ�ա����Ƕ�����CO2������ȼ�ս���������ʵ�飺
| �������� | ʵ������ |
| ������IJ���ȼ�ճ���ȼ�յ���Ѹ�� ���뵽ʢ��װ��CO2�ļ���ƿ�� | ����ʢ��CO2�ļ���ƿ�м���ȼ�� |
| ��Ӧ����ȴ | ����ƿ���ź�ɫ������ƿ���ϸ����а�ɫ ���� |
| ʵ�鲽�� | ʵ������ |
| ��ȡ������ɫ�������Թ��У���������ˮ������Ʒȫ ������ˮ�������м��������CaCl2��Һ | ���ְ�ɫ���� |
| �ھ���Ƭ�̣�ȡ�ϲ���Һ���Թ��У��μ���ɫ��̪��Һ | ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
̼�����׳ƴ������;�ܹ㡣ʵ�����У���̼����狀ͱ���ʳ��ˮ���Ƶô���������ڲ�ͬ�¶��µ��ܽ�ȼ�����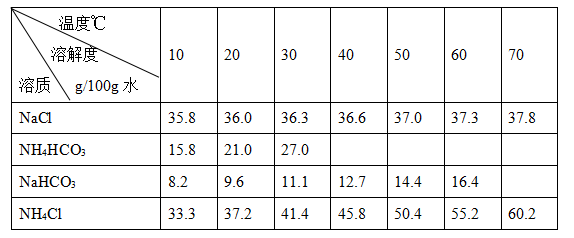
ʵ�鲽��
�����뾫�ƣ��ٴ��Σ���Ca2����Mg2����SO42-���ܽ⣻�ڼ�������NaOH��Na2CO3��Һ����У��۹��ˣ��ܼ��������pH��7��
��ת�����ٽ����ƺ��ʳ����Һ�¶ȿ�����30~35��֮�䣻�ڲ��Ͻ����£�������ϸ��̼����泥����£������Сʱ���ھ��ã�a ��b ���۵õ�NaHCO3���塣
���ƴ�����õ�NaHCO3�����������У��ھƾ��������գ���ȴ�����£����õ����
���������գ�
��1���������뾫�ơ��ɳ�ȥ�Ĵ����е����������� ��
��2����ת���������ӷ���ʽ�� ��
��3����ת���������У��¶ȿ�����30~35��֮��ļ��ȷ�ʽ�� ��Ϊʲô�¶ȿ�����30~35��֮��? ��
��4��a��b���IJ����ֱ��� �� ��
��5��ʵ�����ƵõĴ������NaCl�����ܺ�����NaHCO3��Ϊ�ⶨ����Ĵ��ȣ��õ�����ƽȷ��ȡ��ƷG�ˣ����������ƿ������������ˮ�ܽ⣬�μ�2�η�̪����c mol/L�ı�����ζ�����Һ��dz��ɫ�����ɫ�Ұ���Ӳ��䣬�ζ������������������������������ΪV1mL����ʱ�����ķ�ӦΪ��
CO32-��H����HCO3-
����Ʒ��̼���������ٷֺ����ı���ʽ�� ��
������ƿ��Һ�м����μ�2�μ��ȣ���ͬŨ�ȵ���������ζ����յ㣬������������ΪV2mL���ζ��յ�ʱ��Һ��ɫ�ı仯�� ������ʵ�����ݣ�����ж���Ʒ��NaHCO3 ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������(��Ҫ��Fe2O3��SiO2��Al2O3��MgO������)�Ʊ��������Ĺ����������£�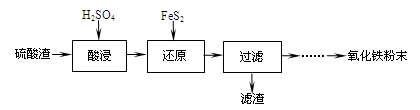
��1���������������Ҫ�ʵ�������Ŀ���ǣ���������Ľ����ʣ��� ��
��2������ԭ���ǽ�Fe3��ת��ΪFe2����ͬʱFeS2������ΪSO42�����÷�Ӧ�����ӷ���ʽΪ
��
��3��Ϊ�ⶨ��������������Һ��Fe3�������Կ��Ƽ���FeS2������ʵ�鲽��Ϊ��
ȷ��ȡһ���������������Һ����ƿ�У�����HCl���Թ���SnCl2���ټ�HgCl2��ȥ������SnCl2���Զ�����������Ϊָʾ������K2Cr2O7����Һ�ζ����йط�Ӧ����ʽ���£�
2Fe3����Sn2����6Cl����2Fe2����SnCl62����
Sn2����4Cl����2HgCl2��SnCl62����Hg2Cl2����
6Fe2����Cr2O72����14H����6Fe3����2Cr3����7H2O��
����SnCl2����������ⶨ��Fe3���� (�ƫ�ߡ�����ƫ�͡��������䡱����ͬ)��
��������HgCl2����ⶨ��Fe3���� ��
��4���ٿ�ѡ�� (���Լ�)������Һ�к���Fe3+������Fe3+��ԭ����
(�����ӷ�Ӧ����ʽ��ʾ)��
����֪����������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Al(OH)3 | Fe(OH)2 | Mg(OH)2 | Mn(OH)2 |
| ��ʼ���� | 2.7 | 3.8 | 7.5 | 9.4 | 8.3 |
| ��ȫ���� | 3.2 | 5.2 | 9.7 | 12.4 | 9.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ������������Ҫ�ɷ���Al2O3��Fe2O3���ͽ�̿�Ʊ���ˮAlCl3���������£�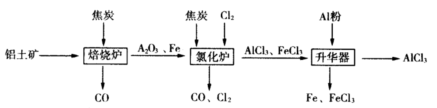
��֪��AlCl3��FeCl3���ֱ���183�桢315������
��1���ڱ���¯�з�����Ӧ��
��Fe2O3(s)��3C(s�� 2Fe(s)��3CO(g�� ��H����492.7kJ/mol
2Fe(s)��3CO(g�� ��H����492.7kJ/mol
��3CO(g)+ Fe2O3(s) 2Fe(s)��3CO2(g�� ��H����25.2kJ/mol
2Fe(s)��3CO2(g�� ��H����25.2kJ/mol
��Ӧ2Fe2O3(s)��3C(s) 4Fe(s)��3CO2(g�� ��H��___________kJ/mol��
4Fe(s)��3CO2(g�� ��H��___________kJ/mol��
��2����Al2O3��Cl2��C���Ȼ�¯�и����·�����Ӧ��������1molAlCl3ʱת��______mol���ӣ�¯���к��д���CO������Cl2������Na2SO3��Һ��ȥCl2�������ӷ���ʽΪ_______�����¶�ԼΪ700�����������м������ۣ�������Ӧ�Ļ�ѧ����ʽΪ ����ַ�Ӧ���¶Ƚ���_____���£��183�桢315��֮һ������ʼ�����ռ�AlCl3��
�ڽ�AlCl3�� 6H2O����Ũ�����������Ҳ�ܵõ�һ��������ˮAlCl3����ԭ��������Ũ�������������е� ������ĸ��ţ���
�������� ����ˮ�� ���ѻӷ��� ����ˮ��
a��ֻ�Т� b��ֻ�Т� c��ֻ�Тڢ� d��ֻ�Тڢۢ�
��3����������������������ʯīΪ�缫���ϣ���ˮΪ�������Һ�����ɵ�ص���������Ӧʽ________����Ǧ������ȣ��ͷ���ͬ����ʱ�������Ľ����缫���ϵ�������m��Al��: m��Pb��=__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����A���ĩ������B��ɵĻ�����ܷ�����ͼ��ʾ��һϵ�з�Ӧ��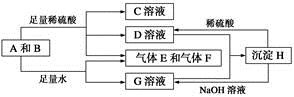
��ش��������⣺
(1)���A���ʵ�Ԫ�������ڱ��д��ڵ�__________����__________�塣
(2)������B�ĵ���ʽΪ_____________________________��
(3)D��G����Һ��Ϻ�����Ӧ�����ӷ���ʽΪ____________________
(4)�����£�D��Һ��pH________7(�����������������)����ԭ����____________________________(�����ӷ���ʽ��ʾ)��
(5)10.8 g A������������NaOH��Һ��Ӧ������������������Ϊ________ g��
(6)��̼����ϡ���ᡢ����E������F���ȼ�ϵ�أ��õ�ص�������ӦʽΪ______________________���Ըõ��Ϊ��Դ���ö��Ե缫���100 g 8%��C��Һ�������ʵ���������Ϊ12.5%ʱֹͣ��⣬��������У����ɵ�������״���µ������Ϊ________ L����·��ͨ�����ӵ����ʵ���Ϊ________ mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����(Na2CO3)�����������о��й㷺����;��������ʵ����ģ���Ƽ�ԭ����ȡNa2CO3������ͼ��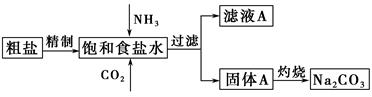
��֪����ʳ��ˮ��ͨ��NH3��CO2�����ķ�ӦΪNaCl��NH3��CO2��H2O=NaHCO3����NH4Cl����ش��������⣺
(1)�����к��е�����������Ca2����Mg2����SO42���ȡ�
���Ƴ��ӵIJ���˳����a�� �� �� ��b(����ĸ���)��
a�������ܽ⣬��ȥ����
b�����������pH
c������Ba(OH)2��Һ
d������Na2CO3��Һ
e������
��ʳ��ˮ����ͨ��NH3����ͨ��CO2�������� ��
(2)���չ���A��Na2CO3�� (����ĸ���)�н��С�
a������ b�������� c���ձ� d����ƿ
֤����ҺA�к���NH4���ķ����� ��
����ҺA�����ؽᾧ�ܹ����NH4HCO3����pH��13��Na����K������Һ�м�������NH4HCO3ʹpH���ͣ���Ӧ�����ӷ���ʽΪ ��
(3)��ͼװ���г�����ʵ�����Ʊ�CO2���� (����ĸ���)����bװ���Ʊ�NH3����Һ©����ʢ�ŵ��Լ��� (���Լ�����)����ƿ�ڿɼ���Ĺ����Լ��� (���Լ�����)��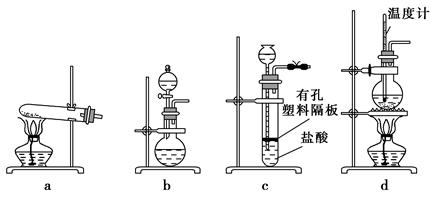
(4)һ����Ȼ���ɷ���aNa2CO3��bNa2SO4��cH2O��ijͬѧ���������ṩ���Լ�����������¼����ⶨNa2CO3������������ʵ�鷽����(������ѡ)���ʵ�鷽����ȫ��
��ѡ����Լ���1.0 mol��L��1 H2SO4��Һ��1.0 mol��L��1 BaCl2��Һ��ϡ��ˮ����ʯ�ҡ�Ca(OH)2��Һ������ˮ
�ٳ�ȡm1g��Ȼ�����Ʒ��������������ˮ�С�
�� ��
�� ��
�ܼ�����Ȼ����к�Na2CO3������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����Ӧ����㷺�Ľ���������±����������Լ������ĺ������ξ�Ϊ��Ҫ�����
(1)Ҫȷ������ij�Ȼ���FeClx�Ļ�ѧʽ���������ӽ����͵ζ��ķ�����ʵ���г�ȡ0.54 g��FeClx��Ʒ���ܽ���Ƚ��������ӽ���Ԥ��������ͨ�����б���OH���������ӽ�������ʹCl����OH������������������ɺ�������Һ��OH����0.40 mol��L��1������ζ��������յ�ʱ��������25.0 mL���������Ʒ���ȵ����ʵ����������FeClx��xֵ��______________________________________ (�г��������)��
(2)����һ����FeCl2��FeCl3�Ļ������Ʒ�����������������n(Fe)��n(Cl)��1��2.1�������Ʒ��FeCl3�����ʵ�������Ϊ________����ʵ�����У�FeCl2�������ۺ�________��Ӧ�Ʊ���FeCl3�������ۺ�________��Ӧ�Ʊ���
(3)FeCl3������ᷴӦʱ��������ɫ���ʣ��÷�Ӧ�����ӷ���ʽΪ________________��
(4)�������(K2FeO4)��һ��ǿ������������Ϊˮ��������������ز��ϡ�FeCl3��KClO��ǿ���������·�Ӧ����ȡK2FeO4���䷴Ӧ�����ӷ���ʽΪ_____________________________����MnO2��Zn������ƣ�K2FeO4��ZnҲ������ɼ��Ե�أ�K2FeO4�ڵ������Ϊ�������ϣ���缫��ӦʽΪ______________________���õ���ܷ�Ӧ�����ӷ���ʽΪ_______________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com