
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�ĵ���У����2011������������¿���ѧ���� ���ͣ�058
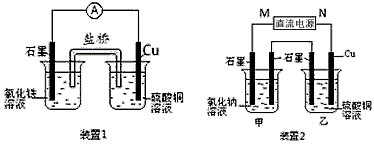
��ͼ��ʾ2��ʵ��װ�ã��ֱ�ش��������⣮
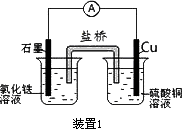
(1)װ��1�е�Cu��________��(���������)����װ�÷������ܷ�Ӧ�����ӷ���ʽΪ________��
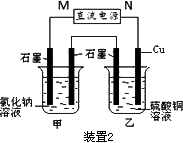
(2)װ��2�м��ձ�ʢ��100 mL��0.2 mol/L��NaCl��Һ�����ձ�ʢ��100 mL��0.5 mol/L��CuSO4��Һ����Ӧһ��ʱ���ֹͣͨ�磮����ձ��е��뼸�η�̪���۲쵽�ұ�ʯī�缫�������ȱ�죬���ʯī�缫��������������
�ٵ�Դ��M��Ϊ________�������ձ��ұ�ʯī�缫�������ȱ���ԭ����________��
�����ձ��е�ⷴӦ�����ӷ���ʽΪ________��
����װ�ü�����������������112 mL����(��״��)����װ����������Һ��pHΪ________(���Է�Ӧǰ����Һ������仯)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��2011ѧ���㽭ʡ����ʦ����ѧ������ѧ�߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
��13�֣������Ǽס��ҡ�����λͬѧ��ȡ���������Ĺ��̡�
��1���ס��ҡ�����λͬѧ����ȡ�Ҵ�����������Ũ�����Ϲ��ȵķ�����ȡ����������Ũ��������������� ��
��2���ס��ҡ�����λͬѧ�ֱ��������ͼ��ʾ����ʵ��װ�ã�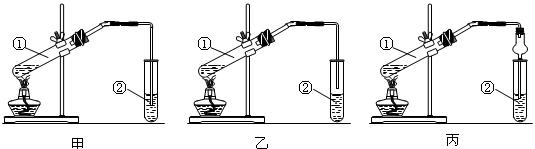 ��Ӽס�����λͬѧ��Ƶ�װ����ѡ��һ����Ϊʵ������ȡ����������װ�ã�Ӧѡ���װ���� ��ѡ��ס����ҡ�������ͬѧ����˱�װ�ã������θ���ܴ��沣���ܣ����������������⣬��һ��Ҫ������ ��
��Ӽס�����λͬѧ��Ƶ�װ����ѡ��һ����Ϊʵ������ȡ����������װ�ã�Ӧѡ���װ���� ��ѡ��ס����ҡ�������ͬѧ����˱�װ�ã������θ���ܴ��沣���ܣ����������������⣬��һ��Ҫ������ ��
��3���Թܢ��б���Na2CO3�����ó����ܽ��Ҵ����������������ܽ���⣬������ ��
��4�����Թܢ��з��������������ʵ������� ��
��5���������������ķ�Ӧ�ǿ��淴Ӧ����Ӧ�ﲻ����ȫ����������Ӧһ��ʱ���
�ʹﵽ�˸÷�Ӧ���ȣ�Ҳ���ﵽ��ѧƽ��״̬������������˵���Ҵ��������������
Ӧ�Ѵﵽ��ѧƽ��״̬����(�����)������������������
�ٵ�λʱ�������1mol����������ͬʱ����1molˮ
�ڵ�λʱ�������1mol����������ͬʱ����1mol����
�۵�λʱ�������1mol�Ҵ���ͬʱ����1mol����
������Ӧ���������淴Ӧ���������
�ݻ�����и����ʵ�Ũ�Ȳ��ٱ仯
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com