
�����ڼ���ȼ�ű��ڻ����������SO2�������ߣ���ɴ�����Ⱦ��ijʵ��С��ͬѧ��̽��SO2�����ʣ����ⶨ������SO2�ĺ�����
��1�������������ʵ��װ�ã��������̽�������ش����⣺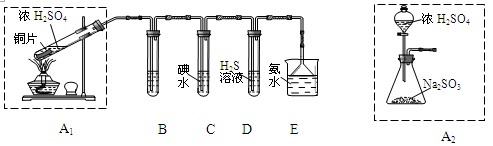
��װ��A1�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��װ��B���ڼ���SO2��Ư���ԣ�������ʢ�Լ�Ϊ ��װ��D���ڼ���SO2�� ���ʣ�
��װ��C�з�Ӧ�����ӷ���ʽΪ ��
��Ϊ��ʵ����ɫ������Ŀ�꣬��ͬѧ����װ��A2����װ��A1������Ϊװ��A2���ŵ��ǣ�д���㣩 �� ��
��2�������������·����ⶨ������SO2�����������������������ԭ�����壩��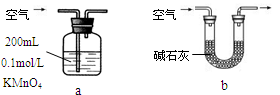
������Ϊ�ĸ�װ�ÿ��У�����ţ� ��ʹ������ѡ�õ�װ�òⶨSO2����ʱ������Ҫ�ⶨ���������� ��
������Ϊ�ĸ�װ�ò����У�����ţ� ��˵������ ��
��1���� Cu + 2H2SO4(Ũ)  CuSO4 + 2H2O + SO2������Ʒ����Һ����������SO2 + I2 + 2H2O ��SO42- + 2I��+ 4H+ �� �ܲ��ü��ȣ���Լ��Դ����ԼҩƷ�������ȫ�����ڿ��Ʒ�Ӧ���У���Ӧ����֣� ��2���� a ����KMnO4��Һ����ɫʱ���ⶨͨ����������V�� �� b �������к��е�CO2Ҳ�����ʯ�ҷ�Ӧ����ɲ�����ȷ��
CuSO4 + 2H2O + SO2������Ʒ����Һ����������SO2 + I2 + 2H2O ��SO42- + 2I��+ 4H+ �� �ܲ��ü��ȣ���Լ��Դ����ԼҩƷ�������ȫ�����ڿ��Ʒ�Ӧ���У���Ӧ����֣� ��2���� a ����KMnO4��Һ����ɫʱ���ⶨͨ����������V�� �� b �������к��е�CO2Ҳ�����ʯ�ҷ�Ӧ����ɲ�����ȷ��
���������������1������װ��A1��Cu��Ũ���Ṳ�ȷ�����Ӧ��Cu + 2H2SO4(Ũ)  CuSO4 + 2H2O + SO2������װ��B���ڼ���SO2��Ư���ԣ�SO2����ijЩ��ɫ������Ʒ�����γ���ɫ�����ʣ����SO2��Ư���ԡ���װ��B��Ʒ����Һ���顣��װ��D�з�����Ӧ��SO2+2H2S=3S��+H2O��SO2�������������������ԣ�H2S�ǻ�ԭ�������ֻ�ԭ�ԡ�����װ��C��SO2���ˮ����I2��SO2��2H2O=H2SO4��2HI����Ӧ�����ӷ���ʽΪ��I2��SO2��2H2O= SO42��+4H++2I-����Ϊ��ʵ����ɫ������Ŀ�꣬��ͬѧ����װ��A2����װ��A1��ʹ��װ��A2���ŵ��Dz��ü��ȣ����Խ�Լ��Դ�����ȫ�����ڿ��Ʒ�Ӧ���У���Ӧ����ֵȣ���2���� ������SO2�Ŀ���ͨ�뵽KMnO4��Һ�У�ֻ��SO2�ܷ�����Ӧ�����Կ��Բⶨ������SO2��������ʹ�����ַ���ֻ��ⶨ��KMnO4��Һ����ɫʱ���ⶨͨ����������V���ɡ�����b�����ڿ����к��е�CO2Ҳ�����ʯ�ҷ�Ӧ��ˮ����Ҳ�ܱ����գ���˻���ɲ�����ȷ���������ڲⶨ������SO2������
CuSO4 + 2H2O + SO2������װ��B���ڼ���SO2��Ư���ԣ�SO2����ijЩ��ɫ������Ʒ�����γ���ɫ�����ʣ����SO2��Ư���ԡ���װ��B��Ʒ����Һ���顣��װ��D�з�����Ӧ��SO2+2H2S=3S��+H2O��SO2�������������������ԣ�H2S�ǻ�ԭ�������ֻ�ԭ�ԡ�����װ��C��SO2���ˮ����I2��SO2��2H2O=H2SO4��2HI����Ӧ�����ӷ���ʽΪ��I2��SO2��2H2O= SO42��+4H++2I-����Ϊ��ʵ����ɫ������Ŀ�꣬��ͬѧ����װ��A2����װ��A1��ʹ��װ��A2���ŵ��Dz��ü��ȣ����Խ�Լ��Դ�����ȫ�����ڿ��Ʒ�Ӧ���У���Ӧ����ֵȣ���2���� ������SO2�Ŀ���ͨ�뵽KMnO4��Һ�У�ֻ��SO2�ܷ�����Ӧ�����Կ��Բⶨ������SO2��������ʹ�����ַ���ֻ��ⶨ��KMnO4��Һ����ɫʱ���ⶨͨ����������V���ɡ�����b�����ڿ����к��е�CO2Ҳ�����ʯ�ҷ�Ӧ��ˮ����Ҳ�ܱ����գ���˻���ɲ�����ȷ���������ڲⶨ������SO2������
���㣺����SO2����ȡ�����顢���ʡ��ⶨ����ѧ����ʽ�����ӷ���ʽ����д��֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��һֻ�Թ���װ��NO2��NO�������a�������³�ѹ����ͬ��������NO2ת��ΪN2O4������������ˮ���У���ˮ��ַ�Ӧ��ʣ������b��������ˮ����������ͨ��c ��O2���Թ��ڸպ�������ʣ�ࡣ
��1��b��ֵΪ ����ֻ��c�Ĵ���ʽ��ʾ����
��2�������
| ����Ҫ�� | ֻ��b�Ĵ���ʽ | ֻ��c�Ĵ���ʽ |
| a��ȡֵ��Χ | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
(22��)������(FeS2)ȼ�ղ�����SO2ͨ�����й��չ��̼����Ƶ�H2SO4�������Ƶ�H2��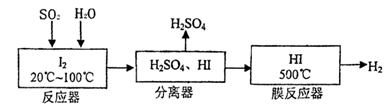
��1���ù�������ѭ�����õ�����Ϊ ��
��2��д����Ӧ���з�����Ӧ�����ӷ���ʽ ��
��3��ij�о���ѧϰС����̽��SO2�ܷ���BaCl2��Һ��Ӧ����BaSO3��������������ʵ�顣
��֪Ũ����ķе�Ϊ338oC������ʱ�ƾ��ƻ�����¶�Ϊ400oC��500oC��
�ټ�ͬѧ��װ��I(����ͼ)����ʵ�飬����BaCl2��Һ�г��ְ�ɫ�������Ұ�ɫ�������������ᣬ��ʵ����۲���˵��SO2����BaCl2��Һ������Ӧ����������ɸð�ɫ���������ֿ���ԭ��(�����ֺ����ӷ���ʽ��ʾ)��
��
��
����ͬѧ�����װ�â�(�г�װ�ú�A�ļ���װ����ȥ)����ʵ�飬����C��û�г��ְ�ɫ������
װ�â�Ľ���װ��I���Ĵ����㣬�����˵�������������
| �Ľ��IJ�����װ��(ע����Ҫ���Լ�) | �Ľ������� |
| ʹ�÷�Һ©���μ�Ũ���� | ���Ʒ�Ӧ���� |
| | |
| | |
| | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
(16��)�������Ʊ���Ϊ��ҵ�Σ���Ư�ס���Ƶȷ���Ӧ�ù㷺����ľ̿��Ũ���ᡢˮ��ͭΪԭ�����ɵ�һ��������������Ʒ�Ӧ�Ʊ��������Ƶ�װ������ͼ��ʾ��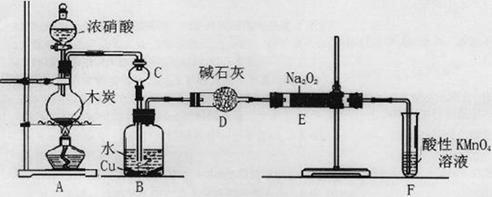
��֪�������£��� 2NO+Na2O2��2NaNO2�������������£�NO��NO2��������MnO4����Ӧ����NO3����Mn2+��5NO2�� + 2MnO4��+ 6H+��5NO3��+ 2Mn2++ 3H2O��
��1��A�й۲쵽������__________________________��
��2��װ��B�з�Ӧ�����ӷ���ʽ��____________________��_____________________��
��3��װ��C�����ã�____________________��װ��F�����ã�___________________��
��4������װ��D����E�в����������������и�����________________________�����ѧʽ��
��5��NaNO2����ʳ��һ������ζ�����������ж�����֪���������ܷ������·�Ӧ��2NaNO2+4HI��2NO+I2+2NaI+2H2O������������Ӧ���������Լ��������г��������ʽ���ʵ�飬�Լ����������ƺ�ʳ�Ρ�����ʵ��ʱ������ѡ�õ�������__________________��
| A������ˮ | B���⻯�ص�����Һ | C������ | D������ E��ʳ�� F���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�о���ѧϰС��ͨ������ʵ��̽��SO2�ܷ���BaCl2��Һ��Ӧ����BaSO3���������Ʊ�����ͭ���塣���������ա���ͬѧ��װ��I����ʵ�飬���ȷ�Ӧ�������ڣ�����BaCl2��Һ�г��ְ�ɫ�������Ұ�ɫ�������������ᡣ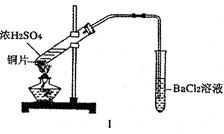
��1����ɫ������ ��
��2����ͬѧ�Ͱ�ɫ����������ԭ����������ּ��裬�����ּ�������ǣ�
�� ��
��ͬѧ����˸Ľ�װ�â����ʵ�飬�����ͬѧ����ļ��裨�г�װ�ú�A�м���װ�����ԣ��������Ѽ��飩��
�ٴ��ɼУ�ͨ��N2һ��ʱ���رյ��ɼУ�
�ڵμ�һ����Ũ���ᣬ����A��һ��ʱ���C��δ���������ɡ�
��3�������ٵ�Ŀ���� ������ƿB�е��Լ��� ��
��4����ʵ���ܷ�����ͬѧ�������ּ����е�����һ�� �������� ��
��ͬѧ��Ӧ�����ɫ��Һ�м���������CuO�����˺���Һ�Ƴ�����ͭ���壨CuSO4��xH2O�������ü��ȷ��ⶨ�þ����нᾧˮx��ֵ��ʵ�����ݼ�¼���£�
| �������� | �����뾧�������� | ���Ⱥ���������������� | |
| ��һ�γ��� | �ڶ��γ��� | ||
| 11.710g | 22.700g | 18.621g | a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������ش�ijͬѧ��̽��Ũ���ᡢϡ���ᡢŨ���ᡢϡ����ֱ���ͭ��Ӧ��ʵ���з��ֵ��й����⡣
��.̽����������������������ǿ��������ͭ��Ӧ�Ļ�ԭ���������
(1)�ֱ���ʢ�е���ͭƬ����֧�Թ��м���������Ũ���ᡢϡ���ᡢŨ���ᡢϡ����,ʵ������¼���±�:
| | �� | ʵ���� |
| a | Ũ���� | ���Ⱥ�����Ӧ,������ɫ�̼������� |
| b | ϡ���� | ����Ҳ��������Ӧ |
| c | Ũ���� | �����ȼ�������Ӧ,��������ɫ���� |
| d | ϡ���� | �ȷ�����Ӧ,������ɫ���� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ��̽����̽��̼����Ԫ�صķǽ����Ե����ǿ��
����Ҫ��������и�С��
��1��ʵ��װ�ã�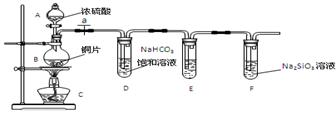
��д��ʾ��������A B
��2��ʵ�鲽�裺
���������� ����ҩƷ��a��Ȼ�����Ũ���ᣬ����
��3������̽��������֪����ǿ��:������ >̼�ᣩ
��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽ�� ��װ��E����������KMnO4��Һ�������� ��
����˵��̼Ԫ�صķǽ����Աȹ�Ԫ�طǽ�����ǿ��ʵ�������� ��
�������Թ�D�е�ʵ�������ܷ�֤����Ԫ�صķǽ�����ǿ��̼Ԫ�صķǽ����� ����ܡ������Թ�D�з�����Ӧ�����ӷ���ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�������ȣ�ClO2���ڳ�������һ�ֻ���ɫ�д̼�����ζ�����壬���۵�Ϊ��59�棬�е�Ϊ11��0�棬������ˮ����ҵ�����Գ�ʪ��KClO3�Ͳ��ᣨH2C2O4����60��ʱ��Ӧ�Ƶá�ijѧ��������ͼ��ʾװ��ģ�ҵ��ȡ���ռ�ClO2��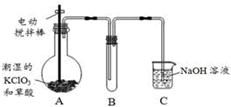
��1��A�з�Ӧ������K2CO3��ClO2��CO2�ȣ���д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��2��A���������¶ȿ���װ�ã����ƾ����⣬����Ҫ�IJ����������ձ��� ��
Bװ�ñ�����ڱ�ˮԡ�У���ԭ���� ��
��3����Ӧ����װ��C�пɵ�NaClO2��Һ����֪NaClO2������Һ���¶ȵ���38��ʱ����������NaClO2��3H2O�����¶ȸ���38��ʱ����������NaClO2���벹���NaClO2��Һ���Ƶ�NaClO2����IJ������裺�� ���� ����ϴ�ӣ��ܸ��
��4��ClO2�ܲ��ȶ������������ƣ�������ˮ���յõ�ClO2��Һ��Ϊ�ⶨ������Һ��ClO2�ĺ���������������ʵ�飺
����1��ȷ��ȡClO2��Һ10��00 mL��ϡ�ͳ�100��00 mL��������ȡV1mL�������뵽��ƿ�У�
����2����ϡ�������������pH��2��0������������KI���壬����Ƭ�̣�
����3���������ָʾ������cmol/LNa2S2O3��Һ�ζ����յ㣬����Na2S2O3��ҺV2mL������֪2 Na2S2O3+ I2��Na2S4O6+ 2NaI��
������100 mL c mol/LNa2S2O3����Һʱ���õ��IJ����������ձ�����Ͳ����������У� ��
�ڵζ��������������������ƽ�вⶨ��ԭ���� ��
��д������2�з�����Ӧ�����ӷ���ʽ ��
��ԭClO2��Һ��Ũ��Ϊ g / L���ò����е���ĸ����ʽ��ʾ����
�����ζ�ǰ�ζ��ܼ����������ݣ��ζ���������ʧ����ⶨ��� ��
���ζ���ʼ���Ӷ������ζ��յ�ʱ��ȷ��������ⶨ��� ��
���ƫ�ߡ���ƫ�͡����䡱 ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ͼ������ʵ���ҽ��а��������Ʊ�������ʵ������װ�ã����̶ֹ�װ��δ������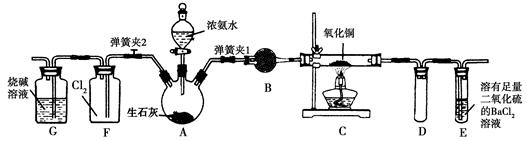
(1)����װ��װ�ú���Ҫ����A��Eװ�õ������ԣ������������________��Ȼ����A���۲쵽E��������ð�����ƿ��ƾ��ƻ��ɿ�˫�֣�E�е�����ˮ���γɣ�˵��װ�����������á�
(2)װ��B��ʢ�ŵ��Լ���________��
(3)��ȼC���ƾ��ƣ��رյ��ɼ�2�����ɼ�1���ӷ�Һ©���ų�Ũ��ˮ����û��ƿ�й����رշ�Һ©�����Ժ�Ƭ�̣�װ��C�к�ɫ������죬װ��E����Һ����ִ������ݣ�ͬʱ����________(������)����E���ݳ�Һ����������ֱ�������������д����C�з�����Ӧ�Ļ�ѧ����ʽ��________________________��
(4)��C�й���ȫ�����ɫ�رյ��ɼ�1�������ƿ��ƾ��ƣ�����ȴ����C�й�������������Ӧǰ��������Ϊ16 g����Ӧ����ع�����������2.4 g��ͨ������ȷ���ù������ijɷ���_________
__________(�û�ѧʽ��ʾ)��
(5)�ڹرյ��ɼ�1���ɼ�2�������������F�У��ܿ췢��װ��F�в������̣�ͬʱ����G����ҺѸ�ٵ�������F�У�д���������̵Ļ�ѧ����ʽ��____________________��Ѹ�ٲ���������ԭ����____________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com