
| NaCl | NaHCO3 | NH4Cl | |
| 10�� | 35.8 | 8.15 | 33.0 |
| 45�� | 37.0 | 14.0 | 50.0 |
���� ��1�����ݷ�Ӧ�ķ���ʽNaCl+NH3+CO2+H2O=NaHCO3+NH4Cl��2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+CO2��+H2O��������Na2CO3��������
��2�������ܽ�ȼ������Ҫ��ˮ����������ȥ��Ӧ��ˮ�����������ռ����ʣ��ˮ�����������ݷ�Ӧ�ķ���ʽ��������NaHCO3������������ܽ�ȼ������������������
��3������ˮ����������ϸ����ʵ��ܽ���ж��������岢����������
��� �⣺��1��117gʳ�ε����ʵ���Ϊn��NaCl��=$\frac{117g}{58.5g/mol}$=2mol��
��NaCl+NH3+CO2+H2O=NaHCO3+NH4Cl
1 1 1
2mol 2mol 2mol
2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+CO2��+H2O
2 1
2mol 1mol
n��Na2CO3��=1mol��
m��Na2CO3��=1mol��106g/mol=106g��
�ʴ�Ϊ��106��
��2��ȡ117gʳ�����Ƴɱ�����Һ����Ҫˮ������Ϊ��$\frac{117g��100}{37}$=316g����Ӧ��ˮ������Ϊ2mol��18g/mol=36g����Һ��ʣ��ˮ������Ϊ��316g-36g=280g��
�ɣ�1���ã�m��NaHCO3��=2mol��84g/mol=168g����ʱ����Һ��ˮ������Ϊ280g���ܽ�NaHCO3������Ϊ$\frac{280}{100}$��14g��39.0g����������������Ϊ168g-39g=129g��
���������������Ϊ129g��
��3���ɣ�1����֪ˮ������Ϊ280g������˳�ȥ������ٽ�����10�棬�ܽ�NaHCO3������Ϊ8.15g��$\frac{280}{100}$=22.8g������NaHCO3������Ϊ39.0-22.8=16.2g��
���ɵ�NH4Cl����Ϊ2mol��53.5g/mol=107g����Һ�ܽ��NH4Cl����Ϊ33.0g��$\frac{280}{100}$=92.4g��������NH4Cl����Ϊ107g-92.4g=14.6g��
�������������ʵ�ΪNH4Cl��NaHCO3��������Ϊ16.2g+14.6g=30.8g��
�����������������Ϊ30.8g��
���� ���⿼�鴿����Ʊ��ͼ��㣬�Լ������������ã���Ŀ�Ѷ��еȣ�ע���й��ܽ�ȼ���ķ����������ڿ���ѧ���ķ��������ͼ���������


 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
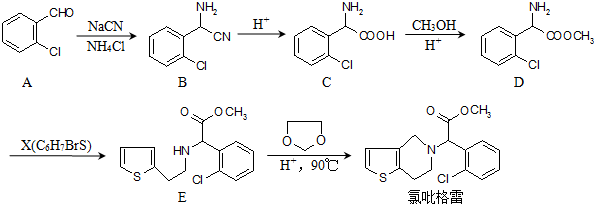
 ��
�� ��
�� ��
��
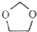 �ĺϳ�·��ͼ�����Լ���ѡ��
�ĺϳ�·��ͼ�����Լ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 3g H2 | B�� | 11.2L HCl | C�� | 1.12L H2O | D�� | 3.01��1023��CH4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | I-131��${\;}_{53}^{77}$I | B�� | Cl-�Ľṹʾ��ͼ��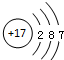 | ||
| C�� | ��Ȳ�Ľṹ��ʽ��CHCH | D�� | Na2S�ĵ���ʽ��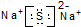 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ʣ���5.44g����Ϊ�� | B�� | ʣ��5.44g����Ϊͭ | ||
| C�� | ������NO���� 0.03mol | D�� | 8.08g������������������Ϊ20.8% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��ѧʽ | ���볣�� |
| HF | Ki=3.5��10-4 |
| H2CO3 | Ki1=4.4��10-7 |
| Ki2=4.7��10-11 | |
| HClO | Ki=3.2��10-8 |
| A�� | ͬ��ͬŨ���£���Һ��pH��NaF��NaClO��Na2CO3 | |
| B�� | ���H+��������ClO-��HCO3-��F- | |
| C�� | ����������Һ��ͨ����������̼�����ӷ���ʽ��ClO-+CO2+H2O�THCO3-+HClO | |
| D�� | ̼������Һ�м����������������ӷ���ʽ��CO32-+2HF�T2F-+H2O+CO2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����������ԭ��Ӧ | B�� | ��˿�ӵ�Դ�ĸ��� | ||
| C�� | ��������������Һ�������� | D�� | ���������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
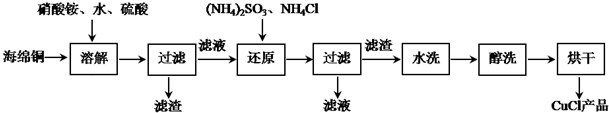
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 18O��31P��119Sn | B�� | 27Al��19F��12C | ||
| C�� | 7N��15P��33As��51Sb��83Bi | D�� | ֻ��һ�����Ӳ��ԭ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com