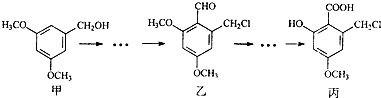
��2012?����һģ���⡢����������Ԫ�ؿ��Էֱ���������絪��������⻯�����������ȣ���ѧ�����Ѿ��о����������������ʿ��������еĹ��ܣ�
��1���£�N
2H
4�����Ʊ�����֮һ�ǽ�NaClO��Һ��NH
3��Ӧ�Ƶã���д���÷�Ӧ�Ļ�ѧ����ʽ
NaClO+2NH3=N2H4+NaCl+H2O
NaClO+2NH3=N2H4+NaCl+H2O
��
��2���¿���Ϊ�����������ȼ�ϣ�NO
2Ϊ����������Ӧ����N
2��ˮ������
N
2+2O
2��g��=2NO
2��g������H=+67.7kJ?mol
-1N
2H
4+O
2��g��=N
2��g��+2H
2O��g������H=-534kJ?mol
-1д���º�NO
2��Ӧ���Ȼ�ѧ����ʽ��
2N2H4��g��+2NO2��g��=3N2��g��+4H2O��g������H=-1135.7kJ?mol-1
2N2H4��g��+2NO2��g��=3N2��g��+4H2O��g������H=-1135.7kJ?mol-1
��
��3����-����ȼ�ϵ����һ�ּ���ȼ�ϵ�أ��������Һ��20%��30%������������Һ���õ�طŵ�ʱ�������ĵ缫��ӦʽΪ
N2H4+4OH--4e-=N2+4H2O
N2H4+4OH--4e-=N2+4H2O
��
��4������������Ʊ������ܶ࣬���з�����ԭ����������ߵ���
D
D
������ţ���
A��BaO
2+H
2SO
4�TBaSO
4��+H
2O
2B��2NH
4HSO
4��NH
4��
2S
2O
8+H
2������NH
4��
2S
2O
8+2H
2O�T2NH
4HSO
4+H
2O
2C��CH
3CHOHCH
3+O
2��CH
3COCH
3+H
2O
2D���һ���������ͼ1
��5�����ݱ��⣨4����Bѡ��ķ�������Ҫ�Ƶ�1mol H
2O
2�����ʱת�Ƶ�����Ϊ
2NA
2NA
��
��6��ij���ױ����˲�ͬ�������Ӽ���Ũ�ȶ�˫��ˮ�������⺣��������Һ��Ӧ���ʵ�Ӱ�죬ʵ������ͼ2��ͼ3��ʾ��
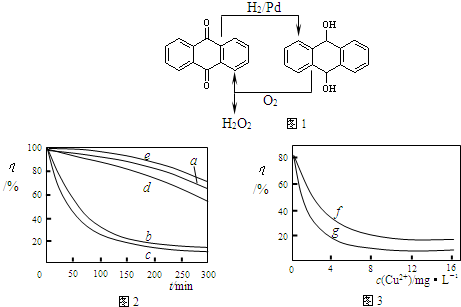
ע������ʵ������¶�Ϊ20�桢w��H
2O
2��=0.25%��pH=7.12������������ҺŨ��Ϊ8mg?L
-1�������½��У�ͼ2������a��H
2O
2��b��H
2O
2+Cu
2+��c��H
2O
2+Fe
2+��d��H
2O
2+Zn
2+��e��H
2O
2+Mn
2+��ͼ3������f����Ӧʱ��Ϊ1h��g����Ӧʱ��Ϊ2h����ͼ�е��������������������Һ��ճ�ȣ���������Ũ������Һճ������أ���
��������Ϣ��֪�����������������
B
B
������ţ���
A����������ʹ�ý��ⷴӦ���ʼ���
B���������ӶԸý��ⷴӦ�Ĵ�Ч�ʱ�ͭ���ӵ�
C������������Һճ�ȵı仯�����ɷ�ӳ���併�ⷴӦ���ʵĿ���
D��һ�������£�ͭ����Ũ��һ��ʱ����Ӧʱ��Խ��������������ҺŨ��ԽС��