
ijĀĮŗĻ½šÖŠŗ¬ÓŠµ„ÖŹĀĮ”¢Ć¾”¢Ķ”¢¹č£¬ĪŖĮĖ²ā¶ØøĆŗĻ½šÖŠĀĮµÄŗ¬Į棬Éč¼ĘĮĖČēĻĀŹµŃéĮ÷³Ģ£¬Ēė»Ų“šÓŠ¹ŲĪŹĢā£ŗ
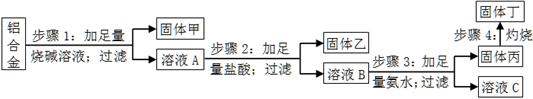
£Ø1£©²½Öč1ĖłµĆ¹ĢĢå¼×µÄ³É·ÖĪŖ ”£
£Ø2£©²½Öč2ÖŠ¼Ó×ćĮæŃĪĖį£¬¶ų²»²ÉÓĆĶØČė×ćĮ涞Ńõ»ÆĢ¼µÄŌŅņĪŖ ”£
£Ø3£©²½Öč3ÖŠÉś³É¹ĢĢå±ūµÄĄė×Ó·½³ĢŹ½ĪŖ £»ČÜŅŗCÖŠĖłŗ¬ÓŠµÄŃōĄė×Ó·ūŗÅĪŖ ”£
£Ø4£©²½Öč4Ėł·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø5£©ĻÖÓĆĶŠÅĢĢģĘ½³ĘČ”10.0gĀĮŗĻ½š½ųŠŠÉĻŹöĮ÷³ĢĶ¼ĖłŹ¾²Ł×÷£¬ĖłµĆ¹ĢĢå¶”ÖŹĮæĪŖ15.3g£¬ŌņøĆĀĮŗĻ½šÖŠĀĮµÄÖŹĮæ·ÖŹżĪŖ £»²¢ÅŠ¶ĻŅŌĻĀ²Ł×÷¶ŌĀĮÖŹĮæ·ÖŹż²ā¶ØµÄÓ°Ļģ£ØĢī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°ĪŽÓ°Ļģ”±£©”£
a£®²½Öč1ÖŠÉÕ¼īĮæ²»×ć£¬Ōņ £»
b£®²½Öč4ÖŠ×ĘÉÕ²»³ä·Ö£¬Ōņ ”£
(16·Ö)
£Ø1£©Ć¾”¢Ķ£Ø2·Ö£©
£Ø2£©ĶØČė¶žŃõ»ÆĢ¼µĆµ½¹čĖįÓėĒāŃõ»ÆĀĮµÄ¹ĢĢå»ģŗĻĪļ£¬ĪŽ·Ø½«¹č”¢ĀĮŌŖĖŲ·ÖĄėæŖĄ“”££Ø2·Ö£©
£Ø3£©Al3+ + 3NH3”¤H2O = Al(OH)3”ż+ 3NH4+£Ø3·Ö£©£»Na+”¢NH4+£Ø2·Ö£©
£Ø4£©2Al(OH)3 Al2O3 + 3H2O£Ø3·Ö£©
Al2O3 + 3H2O£Ø3·Ö£©
£Ø5£©81.0%£Ø2·Ö£©£»Ę«Š”£Ø1·Ö£©£»Ę«“ó£Ø1·Ö£©
”¾½āĪö”æ
ŹŌĢā·ÖĪö£ŗ£Ø1£©Ć¾”¢Ķ²»ÓėÉÕ¼īČÜŅŗ·“Ó¦£¬¹Ź¹ĢĢå¼×µÄ³É·ÖĪŖĆ¾”¢Ķ”£
£Ø2£©ĒāŃõ»ÆĀĮ³ĮµķæÉŅŌŗĶŃĪĖį·“Ó¦£¬¶ų²»ÄÜŗĶ¶žŃõ»ÆĢ¼·“Ó¦£¬¹Ź²»²ÉÓĆĶØČė×ćĮ涞Ńõ»ÆĢ¼µÄŌŅņŹĒĶØČė¶žŃõ»ÆĢ¼µĆµ½¹čĖįÓėĒāŃõ»ÆĀĮµÄ¹ĢĢå»ģŗĻĪļ£¬ĪŽ·Ø½«¹č”¢ĀĮŌŖĖŲ·ÖĄėæŖĄ“”£
£Ø3£©ĒāŃõ»ÆĀĮ³Įµķ²»ŌŁÓė°±Ė®·“Ó¦£¬¹Ź²½Öč3ÖŠÉś³É¹ĢĢå±ūµÄĄė×Ó·½³ĢŹ½ĪŖAl3+ + 3NH3”¤H2O = Al(OH)3”ż+ 3NH4+”£ČÜŅŗCÖŠĖłŗ¬ÓŠµÄŃōĄė×Ó·ūŗÅĪŖNa+”¢NH4+”£
£Ø4£©±ūŹĒĒāŃõ»ÆĀĮ£¬¹Ź·“Ó¦·½³ĢŹ½ĪŖ2Al(OH)3 Al2O3 + 3H2O”£
Al2O3 + 3H2Oӣ
£Ø5£©Al”ś1/2Al2O3
27g 51g
m 15.3g
51g”Įm=27g”Į15.3g
½āµĆm=8.1g
¹Ź10.0gĀĮŗĻ½šÖŠĀĮµÄÖŹĮæ·ÖŹżĪŖ81.0%”£
a£®²½Öč1ÖŠÉÕ¼īĮæ²»×ć£¬Al·“Ó¦²»Ķź£¬¹ŹĘ«Š””£
b£®²½Öč1ÖŠ×ĘÉÕ²»³ä·Ö£¬¶”µÄÖŹĮæ½«Ę«“󣬹ŹĀĮÖŹĮæ·ÖŹż²ā¶ØŅŖĘ«“ó”£
æ¼µć£ŗĢ½¾æĪļÖŹµÄ×é³É»ņ²āĮæĪļÖŹµÄŗ¬Įæ ĀĮµÄ»ÆѧŠŌÖŹ
µćĘĄ£ŗ±¾Ģāæ¼²éѧɜ¶ŌŹµŃéÓė²Ł×÷ŌĄķ”¢ĪļÖŹµÄ·ÖĄėĢį“攢ĪļÖŹŗ¬ĮæµÄ²ā¶Ø”¢»Æѧ¼ĘĖćµČ£¬ÄѶČÖŠµČ£¬Ē峞ŹµŃéŌĄķŹĒ½āĢāµÄ¹Ų¼ü£¬ŹĒ¶ŌĖłŃ§ÖŖŹ¶µÄ×ŪŗĻŌĖÓĆ£¬ŠčŅŖѧɜ¾ß±øŌśŹµµÄ»ł“”ÓėŌĖÓĆÖŖŹ¶·ÖĪöĪŹĢā½ā¾öĪŹĢāµÄÄÜĮ¦”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ĪļÖŹ | Al | Al2O3 | Fe | Fe2O3 |
| ČŪµć/”ę | 660 | 2054 | 1535 | 1462 |
| ·Šµć/”ę | 2467 | 2980 | 2750 | -- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğŗŚĮś½Ź”ĵµ¤½Ņ»ÖŠøßŅ»ĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗŹµŃéĢā
¢ń£®Ä³Ķ¬Ń§×ö”°ĀĮČČ·“Ó¦”±µÄŹµŃ锣²éŌÄ”¶»ÆѧŹÖ²į”·ÖŖ£¬Al”¢Al2O3”¢Fe”¢Fe2O3ČŪµć”¢·ŠµćŹż¾ŻČēĻĀ£ŗ
| ĪļÖŹ | Al | Al2O3 | Fe | Fe2O3 |
| ČŪµć/”ę | 660 | 2054 | 1535 | 1462 |
| ·Šµć/”ę | 2467 | 2980 | 2750 | ”Ŗ”Ŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013Äźø£½ØĻ¼ĘÖŅ»ÖŠøßŅ»ĻĀѧʌµŚŅ»“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗŹµŃéĢā
ijĀĮŗĻ½šÖŠŗ¬ÓŠµ„ÖŹĀĮ”¢Ć¾”¢Ķ”¢¹č£¬ĪŖĮĖ²ā¶ØøĆŗĻ½šÖŠĀĮµÄŗ¬Į棬Éč¼ĘĮĖČēĻĀŹµŃéĮ÷³Ģ£¬Ēė»Ų“šÓŠ¹ŲĪŹĢā£ŗ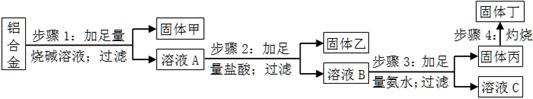
£Ø1£©²½Öč1ĖłµĆ¹ĢĢå¼×µÄ³É·ÖĪŖ ”£
£Ø2£©²½Öč2ÖŠ¼Ó×ćĮæŃĪĖį£¬¶ų²»²ÉÓĆĶØČė×ćĮ涞Ńõ»ÆĢ¼µÄŌŅņĪŖ ”£
£Ø3£©²½Öč3ÖŠÉś³É¹ĢĢå±ūµÄĄė×Ó·½³ĢŹ½ĪŖ £»ČÜŅŗCÖŠĖłŗ¬ÓŠµÄŃōĄė×Ó·ūŗÅĪŖ ”£
£Ø4£©²½Öč4Ėł·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø5£©ĻÖÓĆĶŠÅĢĢģĘ½³ĘČ”10.0gĀĮŗĻ½š½ųŠŠÉĻŹöĮ÷³ĢĶ¼ĖłŹ¾²Ł×÷£¬ĖłµĆ¹ĢĢå¶”ÖŹĮæĪŖ15.3g£¬ŌņøĆĀĮŗĻ½šÖŠĀĮµÄÖŹĮæ·ÖŹżĪŖ £»²¢ÅŠ¶ĻŅŌĻĀ²Ł×÷¶ŌĀĮÖŹĮæ·ÖŹż²ā¶ØµÄÓ°Ļģ£ØĢī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°ĪŽÓ°Ļģ”±£©”£
a£®²½Öč1ÖŠÉÕ¼īĮæ²»×ć£¬Ōņ £»
b£®²½Öč4ÖŠ×ĘÉÕ²»³ä·Ö£¬Ōņ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2015½ģŗŚĮś½Ź”øßŅ»ĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŹµŃéĢā
¢ń£®Ä³Ķ¬Ń§×ö”°ĀĮČČ·“Ó¦”±µÄŹµŃ锣²éŌÄ”¶»ÆѧŹÖ²į”·ÖŖ£¬Al”¢Al2O3”¢Fe”¢Fe2O3ČŪµć”¢·ŠµćŹż¾ŻČēĻĀ£ŗ
|
ĪļÖŹ |
Al |
Al2O3 |
Fe |
Fe2O3 |
|
ČŪµć/”ę |
660 |
2054 |
1535 |
1462 |
|
·Šµć/”ę |
2467 |
2980 |
2750 |
”Ŗ”Ŗ |
ĀĮČČ·“Ó¦·½³ĢŹ½ĪŖ .
ĻĀĮŠÄÜÓėAl·¢ÉśĀĮČČ·“Ó¦µÄÓŠ ”££ØĢīŠņŗÅ£©
AӢMnO2 BӢNa2O CӢMgO DӢFeO
¾ŻÉĻ±ķŹż¾ŻøĆĶ¬Ń§ĶĘ²ā£¬ĀĮČČ·“Ó¦ĖłµĆµ½µÄČŪČŚĪļÓ¦ŹĒĢśĀĮŗĻ½š”£ČōÖ¤Ć÷ÉĻŹöĖłµĆµÄæéדČŪČŚĪļÖŠŗ¬ÓŠ½šŹōĀĮ£¬ĖłÓĆŹŌ¼ĮŹĒ £¬øĆŹŌ¼ĮČÜÖŹµÄµē×ÓŹ½ĪŖ ”£
¢ņ£®Ä³Ķ¬Ń§ĪŖĮĖŃéÖ¤ŗ£“ųÖŠŗ¬ÓŠµā£¬Äā½ųŠŠČēĻĀŹµŃ飬Ēė»Ų“šĻą¹ŲĪŹĢā”£
£Ø1£©µŚ1²½£ŗ×ĘÉÕ”£²Ł×÷ŹĒ½«×ćĮæŗ£“ų×ĘÉճɻŅ½ż”£øĆ¹ż³ĢÖŠ½«Ź¹ÓƵ½µÄŹµŃéŅĒĘ÷ÓŠ
_______________µČ”££ØĢī“śŗÅ£¬ĻĀĶ¬£©
A”¢ŹŌ¹Ü£»B”¢ŪįŪö£»C”¢ÉÕ±£»D”¢Čż½Ē¼Ü£»E”¢ÄąČż½Ē£»F”¢Č÷¾«µĘ£»G”¢Ģś¼ÜĢØ£»H”¢ĮæĶ²
µŚ2²½£ŗI”„ČÜŅŗµÄ»ńČ””£²Ł×÷ŹĒ½«»Ņ½ż×ŖŅʵ½ÉÕ±ÖŠ£¬¼ÓŹŹĮæÕōĮóĖ®£¬ÓĆ²£°ō³ä·Ö½Į°č£¬Öó·Š£¬ĄäČ“£¬________”££ØĢī·ÖĄė·½·Ø£©
£Ø3£©µŚ3²½£ŗŃõ»Æ”£Č”(2)ÖŠČÜŅŗÉŁĮæŅĄ“Ī¼ÓČėŗĻŹŹµÄŹŌ¼Į”£ĻĀĮŠŃõ»Æ¼Į×īŗĆŃ”_________”£
A”¢ÅØĮņĖį B”¢ŠĀÖĘĀČĖ® C”¢KMnO4ČÜŅŗ D”¢H2O2
£Ø4£©µŚ4²½£ŗµāµ„ÖŹµÄ¼ģŃ锣²Ł×÷ŹĒȔɣĮæµŚ3²½µÄČÜŅŗ£¬µĪ¼Ó_______ČÜŅŗ£¬Ö¤Ć÷ŗ£“ųÖŠŗ¬µā”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com