

 £®
£®·ÖĪö ŌŚÄų×÷“߻ƼĮĢõ¼žĻĀ£¬±½ŗĶĒāĘų·¢Éś¼Ó³É·“Ӧɜ³ÉAĪŖ»·¼ŗĶ飬»·¼ŗĶé±»ŃõĘųŃõ»ÆÉś³ÉBĪŖHOOC£ØCH2£©4COOH£¬ŌŚ“߻ƼĮĢõ¼žĻĀ£¬BŗĶ¼×“¼·¢Éśõ„»Æ·“Ӧɜ³ÉCĪŖCH3OOC£ØCH2£©4COOCH3£¬ŌŚŅų×÷“߻ƼĮĢõ¼žĻĀ£¬ŅŅĻ©±»ŃõĘųŃõ»ÆÉś³É»·ŃõŅŅĶ飬»·ŃõŅŅĶéŗĶ°±Ęų·“Ӧɜ³ÉEĪŖHOCH2CH2NH2£¬CŗĶE·“Ӧɜ³ÉF£¬½įŗĻÓŠ»śĪļµÄ½į¹¹ŗĶŠŌÖŹ½ā“š£®
½ā“š ½ā£ŗŌŚŌŚÄų×÷“߻ƼĮĢõ¼žĻĀ£¬±½ŗĶĒāĘų·¢Éś¼Ó³É·“Ӧɜ³ÉAĪŖ»·¼ŗĶ飬»·¼ŗĶé±»ŃõĘųŃõ»ÆÉś³ÉBĪŖHOOC£ØCH2£©4COOH£¬ŌŚ“߻ƼĮĢõ¼žĻĀ£¬BŗĶ¼×“¼·¢Éśõ„»Æ·“Ӧɜ³ÉCĪŖCH3OOC£ØCH2£©4COOCH3£¬ŌŚŅų×÷“߻ƼĮĢõ¼žĻĀ£¬ŅŅĻ©±»ŃõĘųŃõ»ÆÉś³É»·ŃõŅŅĶ飬»·ŃõŅŅĶéŗĶ°±Ęų·“Ӧɜ³ÉEĪŖHOCH2CH2NH2£¬CŗĶE·“Ӧɜ³ÉF£¬
£Ø1£©BŹĒ1£¬6-¼ŗ¶žĖį£¬Ęä½į¹¹¼ņŹ½ĪŖ£ŗHOOC£ØCH2£©4COOH£¬øł¾ŻEµÄ½į¹¹¼ņŹ½ÖŖ£¬EÖŠŗ¬ÓŠōĒ»łŗĶ°±»ł£¬
¹Ź“š°øĪŖ£ŗHOOC£ØCH2£©4COOH£¬ōĒ»łŗĶ°±»ł£»
£Ø2£©CŗĶE·¢ÉśČ”“ś·“Ӧɜ³ÉF£¬·“Ó¦·½³ĢŹ½ĪŖ£ŗCH3OOC£ØCH2£©4COOCH3+2HOCH2CH2NH2 $\stackrel{Ņ»¶ØĢõ¼ž}{”ś}$HOCH2CH2NHOC£ØCH2£©4CONHCH2CH2OH+2CH3OH£¬
¹Ź“š°øĪŖ£ŗCH3OOC£ØCH2£©4COOCH3+2HOCH2CH2NH2 $\stackrel{Ņ»¶ØĢõ¼ž}{”ś}$HOCH2CH2NHOC£ØCH2£©4CONHCH2CH2OH+2CH3OH£»
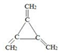
£Ø3£©¾ß±ø¢Łŗ¬ÓŠ3øöĖ«¼ü”¢¢ŚŗĖ“Ź²ÕńĒāĘ×Ö»ĻŌŹ¾1øöĪüŹÕ·åŌņøĆÓŠ»śĪļÖŠÖ»ŗ¬Ņ»ÖÖĄąŠĶµÄĒāŌ×Ó”¢¢Ū²»“ęŌŚ¼×»łµÄ±½µÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½ĪŖ£ŗ £¬
£¬
¹Ź“š°øĪŖ£ŗ £»
£»
£Ø4£©ŅŅĻ©ŌŚŹµŃéŹŅæÉÓÉŅŅ“¼ĶعżĻūČ„·“Ó¦ÖĘČ”£¬
¹Ź“š°øĪŖ£ŗŅŅ“¼£¬ĻūČ„·“Ó¦£»
£Ø5£©a£®AĪŖ»·¼ŗĶ飬»·¼ŗĶéÖŠÖ»“ęŌŚ¹²¼Ūµ„¼ü£¬ĖłŅŌŹōÓŚ±„ŗĶĢž£¬¹ŹÕżČ·£»
b£®»·ŃõŅŅĶéÓėŅŅČ©µÄ·Ö×ÓŹ½¶¼ŹĒC2H4O£¬ĖłŅŌ·Ö×ÓŹ½ĻąĶ¬£¬¹ŹÕżČ·£»
c£®EÖŠŗ¬ÓŠ°±»ł£¬ĖłŅŌÄÜÓėŃĪĖį·“Ó¦£¬¹Ź“ķĪó£»
d£®FÖŠŗ¬ÓŠōĒ»ł£¬ĖłŅŌæÉŅŌ·¢Éśõ„»Æ·“Ó¦£¬¹ŹÕżČ·£»
¹ŹŃ”abd£®
µćĘĄ ±¾ĢāÉę¼°ÓŠ»ś»ÆŗĻĪļÖ®¼äµÄ×Ŗ»Æ¹ŲĻµ”¢¹ŁÄÜĶż°ŠŌÖŹ”¢ÓŠ»ś·“Ó¦ĄąŠĶ”¢ÓŠĢõ¼žµÄĶ¬·ÖŅģ¹¹ĢåµÄŹéŠ“µČĻą¹ŲÖŖŹ¶£¬Ć÷Č·ÓŠ»śĪļµÄ¹ŁÄÜĶż°ĘäŠŌÖŹŹĒ½ā±¾Ģā¹Ų¼ü£¬ÄѶČÖŠµČ£®


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
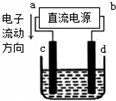
| A£® | aĪŖøŗ¼«”¢bĪŖÕż¼« | B£® | aĪŖŃō¼«”¢bĪŖŅõ¼« | ||
| C£® | µē½ā¹ż³ĢÖŠ£¬ÄĘĄė×ÓÅØ¶Č²»±ä | D£® | µē½ā¹ż³ĢÖŠ£¬dµē¼«ø½½ü±äŗģ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĶźČ«Ńõ»Æ56g FeŠčŅŖĻūŗÄĀČĘų33.6 L | |
| B£® | 7.8g Na2O2ČÜÓŚ100mlĖ®£¬×ŖŅʵē×ÓŹżĪŖ0.1NA | |
| C£® | Ļņ100mL 1mol/LµÄNaHSO3ČÜŅŗÖŠ¼ÓČė×ćĮæµÄĮņĖįĢś£¬Ōņ·“Ó¦ŗóĒāĄė×ÓŌö¼Ó0.4mol | |
| D£® | ĄūÓĆĀĮČČ·“Ó¦½«“ÅĢśæó»¹ŌµĆµ½16.8gĢśµ„ÖŹ£¬·“Ó¦ÖŠFeµĆµ½ĮĖ0.9NAµē×Ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | AlCl3ČÜŅŗÖŠ¼ÓČė¹żĮæ°±Ė®£ŗAl3++4NH3•H2OØTAlO${\;}_{2}^{-}$+4NH${\;}_{4}^{+}$+2H2O | |
| B£® | ŌŚŗ£“ų»ŅµÄ½ž³öŅŗ£Øŗ¬ÓŠI-£©ÖŠµĪ¼ÓH2O2µĆµ½I2£ŗ2I-+H2O2+2H+ØTI2+O2”ü+2H2O | |
| C£® | ŌŚĒāŃõ»Æ±µČÜŅŗÖŠµĪ¼ÓĮņĖįĒā¼ŲČÜŅŗÖĮPH=7£ŗBa2++2OH-+2H++SO42-ØTBaSO4”ż+2H2O | |
| D£® | ÓĆĢ¼ĖįÄĘČÜŅŗ½žÅŻ¹ųĀÆĖ®¹ø£ŗCa2++CO32-ØTCaCO3”ż |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĖüÓŠĄūÓŚ¶łĶÆŃĄ³ŻµÄÉś³¤ | |
| B£® | ĖüÓŠĄūÓŚ¹Ē÷ĄµÄÉś³¤ŗĶ·¢Óż | |
| C£® | ĖüÓŠĄūÓŚ»ÆŗĻĪļCa10£ØPO4£©6£ØOH£©2µÄÉś³É | |
| D£® | ĖüŹ¹¶łĶÆøü“ĻĆ÷ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com