
| V(±ź×¼)”Įc(±ź×¼) |
| V(“ż²ā) |
| V(±ź×¼)”Įc(±ź×¼) |
| V(“ż²ā) |
| V(±ź×¼)”Įc(±ź×¼) |
| V(“ż²ā) |
| V(±ź×¼)”Įc(±ź×¼) |
| V(“ż²ā) |
| V(±ź×¼)”Įc(±ź×¼) |
| V(“ż²ā) |


 Źī¼Ł×÷ŅµŹī¼ŁæģĄÖĮ·Ī÷°²³ö°ęÉēĻµĮŠ“š°ø
Źī¼Ł×÷ŅµŹī¼ŁæģĄÖĮ·Ī÷°²³ö°ęÉēĻµĮŠ“š°ø ŠĀ»īĮ¦×ܶÆŌ±ŹīĻµĮŠ“š°ø
ŠĀ»īĮ¦×ܶÆŌ±ŹīĻµĮŠ“š°ø ĮśČĖĶ¼ŹéæģĄÖ¼ŁĘŚŹī¼Ł×÷ŅµÖ£ÖŻ“óѧ³ö°ęÉēĻµĮŠ“š°ø
ĮśČĖĶ¼ŹéæģĄÖ¼ŁĘŚŹī¼Ł×÷ŅµÖ£ÖŻ“óѧ³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| AӢH2SO3?2H++SO32- |
| BӢHF=H++F- |
| CӢNaHS=Na++H++S2- |
| DӢH2CO3+H2O?H3O++HCO3- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢¢Ł¢Ż | B”¢¢Ś¢Ü¢Ż |
| C”¢¢Ś¢Ū¢Ü¢Ż | D”¢¢Ł¢Ś¢Ū¢Ü¢Ż |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
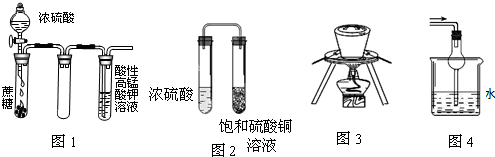
| A”¢Ķ¼1ĖįŠŌKMnO4ČÜŅŗ֊ƻӊĘųÅŻ³öĻÖ£¬µ«ČÜŅŗŃÕÉ«»įÖš½„±äĒ³ÄĖÖĮĶŹČ„ |
| B”¢Ķ¼2¾ĆÖĆŗ󣬱„ŗĶĮņĖįĶČÜŅŗæÉÄÜĪö³öĄ¶É«¾§Ģå |
| C”¢Ķ¼3ŌŚŗ£“ųĢįµāŹµŃéÖŠÓĆÓŚ×ĘÉÕŗ£“ų |
| D”¢Ķ¼4æÉÓĆÓŚĪüŹÕŅ×ČÜÓŚĖ®µÄĪ²Ęų |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
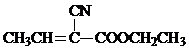 £©ŹĒŗĻ³ÉijÖÖŹÖŹõÓĆÕ³ŗĻ¼ĮµÄµ„Ģ壬XµÄŗĻ³ÉĀ·ĻßČēĶ¼£ŗ
£©ŹĒŗĻ³ÉijÖÖŹÖŹõÓĆÕ³ŗĻ¼ĮµÄµ„Ģ壬XµÄŗĻ³ÉĀ·ĻßČēĶ¼£ŗ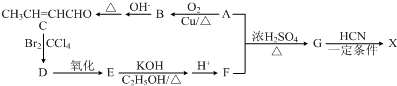

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
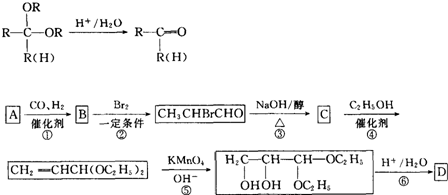
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢ĻņFeCl2 ČÜŅŗÖŠÖšµĪ¼ÓČėNaOHČÜŅŗ |
| B”¢ĻņFeSO4ČÜŅŗÖŠÖšµĪ¼ÓČė°±Ė® |
| C”¢ĻČ½«Ź¢ÓŠNaOHČÜŅŗµÄ³¤µĪ¹Ü²åµ½FeSO4ŅŗĆęĻĀ£¬ŌŁ¼·³öNaOHČÜŅŗæÉÖʵĆFe£ØOH£©2µÄ°×É«³Įµķ |
| D”¢Č”ŠĀÅäÖʵÄFeSO4ČÜŅŗŹŹĮæ·ÅČėŹŌ¹ÜÖŠ£¬ŌŚ¼ÓČėŅ»²ćÖ²ĪļÓĶ£ØĆܶȊ”ÓŚĖ®£¬ĒŅ²»ČÜÓŚĖ®£©£¬Č»ŗóĻņŹŌ¹ÜÄŚÖšµĪ¼ÓČėNaOHČÜŅŗ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com