


 (1+b)��
(1+b)��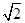 ��1+a�� ��2�֣�
��1+a�� ��2�֣�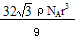 ��2�֣�
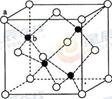
��2�֣�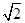 ��1+a��r��CsCl����Ϊ�����������ṹ������y2+2y2=��2r+2br��2���ɵ�y=2/
��1+a��r��CsCl����Ϊ�����������ṹ������y2+2y2=��2r+2br��2���ɵ�y=2/ (1+b)r��x��y=
(1+b)r��x��y= (1+b)��
(1+b)��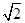 ��1+a��
��1+a�� /3r ��ͼ���������V=a3=64
/3r ��ͼ���������V=a3=64 /9r3�����ݾ�̯����֪���о�����Feԭ�ӣ�8��1/8+1=2,��Fe�����ԭ������ΪM����64
/9r3�����ݾ�̯����֪���о�����Feԭ�ӣ�8��1/8+1=2,��Fe�����ԭ������ΪM����64 /9r3?��="2M/" NA,M=
/9r3?��="2M/" NA,M=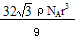



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 Ϊ̼ԭ�ӣ�
Ϊ̼ԭ�ӣ�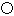 Ϊ��ԭ��)��ÿ��̼ԭ����Χ�����������Ĺ�ԭ����________�����辧���߳�Ϊa cm���ܶ�Ϊb g��cm��3�����ӵ������ɱ�ʾΪ________(�ú�a��b��ʽ�ӱ�ʾ)��
Ϊ��ԭ��)��ÿ��̼ԭ����Χ�����������Ĺ�ԭ����________�����辧���߳�Ϊa cm���ܶ�Ϊb g��cm��3�����ӵ������ɱ�ʾΪ________(�ú�a��b��ʽ�ӱ�ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��һ���ڻ�ѧʵ��Ϳ�ѧ�о��У�ˮ��һ����õ��ܼ���ˮ������֮Դ���������ǵ�����������ء�
��һ���ڻ�ѧʵ��Ϳ�ѧ�о��У�ˮ��һ����õ��ܼ���ˮ������֮Դ���������ǵ�����������ء�| A����ԭ�ӵ��ӻ����ͷ����˸ı� | B��������״�����˸ı� |
| C�����Ļ�ѧ���ʷ����˸ı� | D�����еļ��Ƿ����˸ı� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
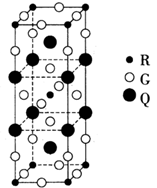
| Ԫ�� | �й����ʼ��ṹ��Ϣ | |
| A | A��һ��ԭ���������� | |
| B | Bԭ�ӵĵ��������4��ԭ�ӹ�� | |
| C | C22��������縺������Ԫ�صĵ��ʻ�Ϊ�ȵ����� | |
| D | D�Ƕ����ڽ�������ǿ��Ԫ�� | |
| E | C��Eͬ�� | |
| F | ��Χ�����Ų�ʽΪnd2n(n+1)s(n-1) | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2NH3ʵ�ִ�������⡣����˵����ȷ���� ������ѡ��
2NH3ʵ�ִ�������⡣����˵����ȷ���� ������ѡ��


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com