
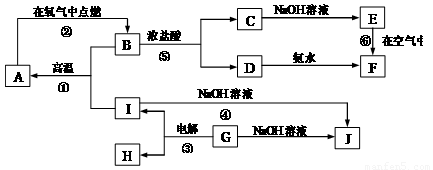
�� 10�� ��A~I�ֱ��ʾ��ѧ��ѧ�г�����һ�����ʣ�����A��IΪ��������������֮������ϵ��ͼ��ʾ�����ַ�Ӧ�������û���г���������֪GΪ����Ԫ�صĹ�̬�����A��B��C��D��E��F���������о���ͬһ��Ԫ�أ�F�Ǻ��ɫ���塣
����д���пհף�
��1��A��B��C��D��E��F����������������ͬһ��Ԫ���� ��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ�����ӷ���ʽ��
��Ӧ�ٵĻ�ѧ����ʽ�� ��
��Ӧ�ܵ����ӷ���ʽ�� ��
��Ӧ�Ļ�ѧ����ʽ�� ��
��3���������仯�ĽǶȿ�����Ӧ�٢ڢ��У����ڡ�H��0�ķ�Ӧ�� ������ţ���

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�� 10�� ��A~I�ֱ��ʾ��ѧ��ѧ�г�����һ�����ʣ�����A��IΪ��������������֮������ϵ��ͼ��ʾ�����ַ�Ӧ�������û���г���������֪GΪ����Ԫ�صĹ�̬�����A��B��C��D��E��F���������о���ͬһ��Ԫ�أ�F�Ǻ��ɫ���塣
����д���пհף�
��1��A��B��C��D��E��F����������������ͬһ��Ԫ���� ��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ�����ӷ���ʽ��
��Ӧ�ٵĻ�ѧ����ʽ�� ��
��Ӧ�ܵ����ӷ���ʽ�� ��
��Ӧ�Ļ�ѧ����ʽ�� ��
��3���������仯�ĽǶȿ�����Ӧ�٢ڢ��У����ڡ�H��0�ķ�Ӧ�� ������ţ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ݷ��ʵ����и����������¿���ѧ�Ծ� ���ͣ������
�� 10�� ��A~I�ֱ��ʾ��ѧ��ѧ�г�����һ�����ʣ�����A��IΪ��������������֮������ϵ��ͼ��ʾ�����ַ�Ӧ�������û���г���������֪GΪ����Ԫ�صĹ�̬�����A��B��C��D��E��F���������о���ͬһ��Ԫ�أ�F�Ǻ��ɫ���塣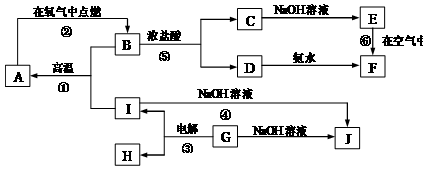
����д���пհף�
��1��A��B��C��D��E��F����������������ͬһ��Ԫ���� ��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ�����ӷ���ʽ��
��Ӧ�ٵĻ�ѧ����ʽ�� ��
��Ӧ�ܵ����ӷ���ʽ�� ��
��Ӧ�Ļ�ѧ����ʽ�� ��
��3���������仯�ĽǶȿ�����Ӧ�٢ڢ��У����ڡ�H��0�ķ�Ӧ�� ������ţ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�������ʡ���������ο��ԣ����ۣ���ѧ���� ���ͣ������
��(10��)��A��B��C��D��E���ֶ�����Ԫ�أ����ǵ�ԭ��������������AԪ�ص�ԭ���ǰ뾶��С��ԭ�ӡ�BԪ�ص�����������ˮ���������⻯�ﷴӦ����һ����X��D��Aͬ�壬����Eͬ���ڣ�EԪ�ص������������Ǵ�����������3��4����A��B��D��E������Ԫ�أ�ÿһ�ֶ�����CԪ���γ�ԭ�Ӹ����Ȳ���ͬ�Ķ��ֻ�����ش��������⣺
(1)д����ӦԪ�ط��ţ�A B C E
(2)��A��C��D��E����Ԫ������ɵ�һ����ѧ�����Ļ���������������ᷴӦ��������NaOH��Һ��Ӧ�������ֻ������ˮ��Һ�У�������ɫʯ����Һ��Һ�ʺ�ɫ������Һ������Ũ���ɴ�С������˳��Ϊ�� ��
(3)����Ƭ��þƬ������A��C��D����Ԫ��������ʵ�ϡ��Һ�й���ԭ��أ����ĵ缫��ӦʽΪ ��
 ��(5��)��֪X��һ���Σ�H�dz����������ʣ�F��I�dz����ǽ������ʣ�E��G���ǹ�ҵ����Ҫ�ļ������ʣ���������ͼ��ʾ�Ĺ�ϵ��
��(5��)��֪X��һ���Σ�H�dz����������ʣ�F��I�dz����ǽ������ʣ�E��G���ǹ�ҵ����Ҫ�ļ������ʣ���������ͼ��ʾ�Ĺ�ϵ��
�Իش���������
(1)G�Ļ�ѧʽΪ��
(2)д�����з�Ӧ�Ļ�ѧ����ʽ
��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(10��)��A��B��C�ֱ��ʾ����10�����ӵ���(����),D��E��F��G�ֱ��ʾ����18�����ӵ���(����),��ش�:
(1) ����A��D��ͬϵ���D���ȴ����� �ֽṹ
(2) B��E������ͬ��Ԫ�أ�B��ˮ��Һ�ʼ��ԣ�E��һ�ֻ��ȼ�ϵijɷ֣���E�ĵ���ʽ
(3) C��F������ͬԪ�أ�д��F��I-��Ӧ�����ӷ�Ӧ����ʽ
(4) G��������Ԫ����ɵ���ԭ�ӷ��ӣ���8.0g NaOH����Һ��ͨ��һ����G���õ�����ҺС�����ɣ��Ƶ���ˮ��7.9g�������ˮ����һ�����е������� ���ѧʽ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com