

���� ��1����Ag2TeҲ����O2��������Cu2Te�ķ�Ӧ�����Cu2Te��������Ӧ���ص���д��
�ڹ�ҵ��һ����ѹ������ѹ��ԭ������ѹ��
��2�����������ڷ�������Һ�壻
��3���ɲ������֪�����Ag��Au�����ڽ���Һ�������ʺ���TeOSO4��CuSO4��
��4�����Һ��ͭ����С������ʼ������Ӧ����ͭ��
��5����TeOSO4�����������������ԭ��Ӧ����Te��
��NaCl��Դ�ḻ���۸���ˣ�
�۸���Te��IV����Ũ�ȵı仯���㻹ԭ�ʣ�
��� �⣺��1����Ag2TeҲ����O2��������Cu2Te�ķ�Ӧ������������Ӧ����Ag2O��TeO2����Ӧ�Ļ�ѧ����ʽΪ2Ag2Te+3O2=2Ag2O+2TeO2��
�ʴ�Ϊ��2Ag2Te+3O2=2Ag2O+2TeO2��
�ڹ�ҵ��һ����ѹ������ѹ��ԭ������ѹ���ʴ�Ϊ����ѹ������ѹ��
��2�����������ڷ�������Һ�壬Ϊ���˲������ʴ�Ϊ�����ˣ�
��3���ɲ������֪�����Ag��Au�����ڽ���Һ�������ʺ���TeOSO4��CuSO4���ʴ�Ϊ��CuSO4��
��4�����Һ��ͭ����С������ʼ������Ӧ����ͭ���缫����ʽΪCu2++2e-=Cu���ʴ�Ϊ��Cu2++2e-=Cu��
��5����TeOSO4�����������������ԭ��Ӧ����Te����Ӧ�Ļ�ѧ����ʽΪTeOSO4+2SO2+3H2O=Te+3H2SO4��
�ʴ�Ϊ��TeOSO4+2SO2+3H2O=Te+3H2SO4��
��NaCl��Դ�ḻ���۸���ˣ��ʴ�Ϊ��NaCl��KI�۸���ˣ�
��Te��IV��Ũ�ȴ�6.72g•L-1�½���0.10g•L-1��Te��IV���Ļ�ԭ��Ϊ$\frac{6.72-0.10}{6.72}��100%$=98.5%��
�ʴ�Ϊ��98.5%��
���� ����Ϊ���������⣬Ϊ�߿��������ͺ�Ƶ���㣬�漰�����Ļ��ա�������ԭ��Ӧ�����ʵķ����ᴿ�ͳ��ӣ�������ѧ���ķ��������������Ŀ��飬��Ŀ��Ϊ�ۺϣ�����ʱע����ϸ���⣬����Ŀ�л�ȡ�ؼ���Ϣ�������ѶȽϴ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��NaH��H2O��Ӧ�Ļ�ѧ����ʽΪNaH+H2O�TNaOH+H2����
��NaH��H2O��Ӧ�Ļ�ѧ����ʽΪNaH+H2O�TNaOH+H2�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
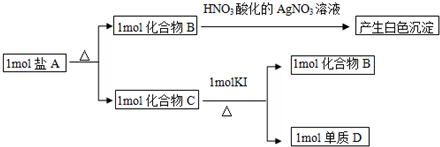
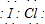 ���û������������۵���IԪ�أ�
���û������������۵���IԪ�أ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����·����1.204��1022������ת��ʱ�����ձ�����Һ��C��H+��ԼΪ0.1 mol•L-1 | |
| B�� | ��ع���ʱ�������е�K+������ձ� | |
| C�� | ��ع���ʱ�����·�ĵ��ӷ����Ǵ�a��b | |
| D�� | �ҳ��е���������ΪSO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H${\;}_{2}^{18}$O��Ͷ��Na2O2���壺2H${\;}_{2}^{18}$O+2O${\;}_{2}^{2-}$�T4OH-+18O2�� | |
| B�� | ��0.1 mol•L-1��pH=1��NaHA��Һ�м���NaOH��Һ��H++OH-�TH2O | |
| C�� | �Խ�����Ϊ������ⱥ������ͭ��Һ��Cu2++2H2O�T2Cu+O2��+4H+ | |
| D�� | NH4Al��SO4��2��Һ�м���Ba��OH��2��ҺSO42-ʹ��ȫ������Al3++2SO42-+2Ba2++4OH-�TAlO2-+2BaSO4��+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
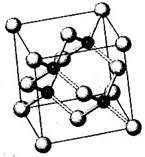 N��P��As��Ga��Cr��Ԫ�ػ���������࣬������Ҫ���о���ֵ��Ӧ�ü�ֵ����ش��������⣺
N��P��As��Ga��Cr��Ԫ�ػ���������࣬������Ҫ���о���ֵ��Ӧ�ü�ֵ����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ���õ���ʽ��ʾ����
���õ���ʽ��ʾ�����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com