
���� ��1��ag����Fe�����ʵ�����ȣ�Fe�����ʵ���Ϊ1mol�����ݷ���ʽ������������������ʵ������ٸ�����ͬ���������֮�ȵ������ʵ���֮��ȷ������Ӧ�������������֮�ȣ�
��2��CO2��H2�Ļ�����壬ͨ���������ȵ�Fe3O4�����е�H2��Fe3O4��Ӧ����Fe��H2O�����������ɣ���ʣ������nL��ΪCO2����ԭ������H2�����Ϊ��m-n��L��
��3����3Fe+8HNO3 =3Fe��NO3��2+4H2O+2NO���������������������NO���������Һ�ijɷ�ΪFe��NO3��2������ϡ�����൱��������H+����Fe��NO3��2�е�NO-3����������ᣬ�ٴη���3Fe+8HNO3 =3Fe��NO3��2+4H2O+2NO�����ݴ˽��з�����
��� �⣺��1��ag����Fe�����ʵ�����ȣ���Fe�����ʵ���Ϊ1mol����
Fe+H2SO4=FeSO4+H2��
1mol 1mol
3Fe+4H2O��g��$\frac{\underline{\;����\;}}{\;}$Fe3O4+4H2
3mol 4mol
1mol $\frac{4}{3}$mol
��ͬ���������֮�ȵ������ʵ���֮�ȣ�������Ӧ�������������֮��Ϊ1mol��$\frac{4}{3}$mol=3��4��
�ʴ�Ϊ��3��4��
��2��CO2��H2�Ļ�����壬ͨ���������ȵ�Fe3O4�����е�H2��Fe3O4��Ӧ����Fe��H2O�����������ɣ���ʣ������nL��ΪCO2����ԭ������H2�����Ϊ��m-n��L��
�ʴ�Ϊ��m-n����
��3��n��HNO3��=0.8L��1mol•L-1=0.8mol
3Fe+8HNO3 =3Fe��NO3��2+4H2O+2NO��
8 3 2
0.8 0.3 0.2
��n��Fe��NO3��2��=0.3mol��n��NO��=0.2mol����V��NO��=0.2mol��22.4L/mol=4.48L��
n��Fe��NO3��2��=0.3mol������Ԫ���غ���n��NO-3��=0.6mol��
n��H2SO4��=0.1L��1mol•L-1=0.1mol����n��H+��=0.2mol��
����������Һ�ijɷ�ΪFe��NO3��2������ϡ�����൱��������H+����Fe��NO3��2�е�NO-3����������ᣬ
��3Fe+8H++2NO-3=3Fe2++4H2O+2NO����ע�����ʱ�Բ������Ϊ�������
8 2
0.2 0.05
��n��NO��=0.05mol����V��NO��=0.05mol��22.4L/mol=1.12L��
�ʴ�Ϊ��4.48��1.12��
���� ���⿼��ѧ�����÷���ʽ���м��㣬ע��Ԫ���غ��Ӧ�ã������ѵ㣨3������ϡ�����൱��������H+������Fe��NO3��2�е�NO3-����������ᣬҲ���ܽ������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
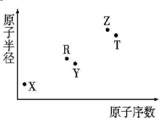 ����������Ԫ��X��Y��Z��R��T��ԭ�Ӱ뾶��ԭ��������ϵ��ͼ��ʾ��R��X���γ�X2R��X2R2�����Z�ǵؿ��к������Ľ���Ԫ�أ�Tԭ��������������K���������2���������ƶ���ȷ���ǣ�������
����������Ԫ��X��Y��Z��R��T��ԭ�Ӱ뾶��ԭ��������ϵ��ͼ��ʾ��R��X���γ�X2R��X2R2�����Z�ǵؿ��к������Ľ���Ԫ�أ�Tԭ��������������K���������2���������ƶ���ȷ���ǣ�������| A�� | X��Y��ɵĻ������ˮ��Һ����������ƿ�� | |
| B�� | ԭ�Ӱ뾶�����Ӱ뾶�����㣺Y��Z | |
| C�� | ����������Ӧ��ˮ�������ԣ�Z��T | |
| D�� | ��ZԪ�ص�����Һһ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ƭ��ϡ������ȡ����ʱ������NaNO3�����Na2SO4���嶼��Ӱ���������������� | |
| B�� | ���뷴Ӧ���λ����ڻ���Ӱٷ�������ѧ��Ӧ�������� | |
| C�� | ����β����NO��CO���Ի�����Ӧ����N2��CO2����Сѹǿ��Ӧ���ʼ��� | |
| D�� | �� 0.1 mol•L-1NH4A1��SO4��2 ��Һ�� 0.3 mol•L-1Ba��OH��2��Һ�������ϣ�Al3++2SO42-+2Ba2++40H-�TAlO2-+2BaSO4��+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
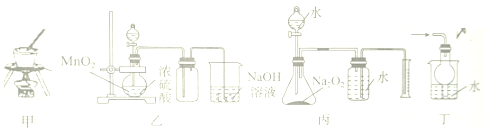
| A�� | ���ü�װ�ü״Ӻ�ˮ����ȡNaCl���� | |
| B�� | ����װ�����Ʊ����ռ�Cl2 | |
| C�� | ����װ�ñ��ⶨ����Na2O2�Ĵ��� | |
| D�� | ����װ�ö�����HClβ������ֹҺ�嵹�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
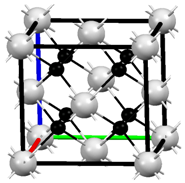 M��R��X��YΪԭ��������������Ķ���������Ԫ�أ�Z��һ�ֹ���Ԫ�أ�M��̬ԭ��L����p�����������s�����������2����R��ͬ����Ԫ��������õĽ���Ԫ�أ�X��M�γɵ�һ�ֻ������������������Ҫ������Ⱦ��û�������Ư���ԣ���ʹƷ����Һ��ɫ��Z�Ļ�̬ԭ��4s��3d������������ش��������⣺
M��R��X��YΪԭ��������������Ķ���������Ԫ�أ�Z��һ�ֹ���Ԫ�أ�M��̬ԭ��L����p�����������s�����������2����R��ͬ����Ԫ��������õĽ���Ԫ�أ�X��M�γɵ�һ�ֻ������������������Ҫ������Ⱦ��û�������Ư���ԣ���ʹƷ����Һ��ɫ��Z�Ļ�̬ԭ��4s��3d������������ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
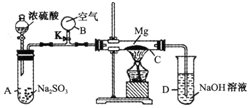 ����ͼװ�ý�������ʵ�飬��������ѧ֪ʶ������⣺�ȹر�K��ʹA�з�Ӧ���У����Ȳ�����C���ɹ۲쵽C���з���ҫ�۰⣬�������̣��ܱ��ϸ��е���ɫ���ʣ�ʵ����ɺ�C���й���ȫ�����������У��г�������ζ���������ɣ�
����ͼװ�ý�������ʵ�飬��������ѧ֪ʶ������⣺�ȹر�K��ʹA�з�Ӧ���У����Ȳ�����C���ɹ۲쵽C���з���ҫ�۰⣬�������̣��ܱ��ϸ��е���ɫ���ʣ�ʵ����ɺ�C���й���ȫ�����������У��г�������ζ���������ɣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
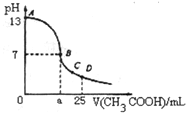 ��������25mL0.1mol/LNaOH��Һ����μ���0.2mol/LCH3COOH��Һ��������ͼ��ʾ���й�����Ũ�ȹ�ϵ�Ƚ���ȷ���ǣ�������
��������25mL0.1mol/LNaOH��Һ����μ���0.2mol/LCH3COOH��Һ��������ͼ��ʾ���й�����Ũ�ȹ�ϵ�Ƚ���ȷ���ǣ�������| A�� | ��A��B������һ�㣬��Һ��һ������c��Na+��+c��H+���Tc��CH3COO-��+c��OH-�� | |
| B�� | ��B�㣺a��12.5������c��Na+���Tc��CH3COO-��=c��OH-���Tc��H+�� | |
| C�� | ��C�㣺c��Na+����c��CH3COO-����c��H+����c��OH-�� | |
| D�� | ��D�㣺c��CH3COO-��=c��CH3COOH�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com