
·ÖĪö £Ø1£©ÓÉ”°ÄÉĆ×¼¼Źõ”±ŹĒÖøĮ£×ÓÖ±¾¶ŌŚ¼øÄÉĆ×µ½¼øŹ®Ć׵IJÄĮĻ£¬Ōņ·ÖÉ¢µ½ŅŗĢå·ÖÉ¢¼ĮÖŠ£¬·ÖÉ¢ÖŹµÄÖ±¾¶ŌŚ1nm”«100nmÖ®¼ä£»
£Ø2£©¢ŁĖäČ»Ķā¹ŪĻąĶ¬µÄĖ®ČÜŅŗŗĶ½ŗĢåÓŠŗܶąŠŌÖŹ²īŅģ£¬µ«ÓĆÓŚĒų±š¶žÕß×ī¼ņ±ćæÉææµÄĢŲÕ÷ŠŌÖŹ»¹ŹĒ¶”“ļ¶ūĻÖĻó£»
¢Śµķ·ŪŌŚµķ·ŪĆøµÄ×÷ÓĆĻĀ»įĖ®½ā³ÉŠ”·Ö×Ó£¬æÉĶø¹ż°ėĶøĤ£¬µ«Ćø×÷ĪŖµ°°×ÖŹĮōŌŚ°ėĶøĤ“üÄŚ£®
½ā“š ½ā£ŗ£Ø1£©É¢ĻµÖŠ·ÖÉ¢ÖŹµÄÖ±¾¶ŌŚ1nm”«100nmÖ®¼äµÄŹōÓŚ½ŗĢå·ÖÉ¢Ļµ£¬ÓÉ”°ÄÉĆ×¼¼Źõ”±ŹĒÖøĮ£×ÓÖ±¾¶ŌŚ¼øÄÉĆ×µ½¼øŹ®ÄÉĆ׵IJÄĮĻ£¬Ōņ·ÖÉ¢µ½ŅŗĢå·ÖÉ¢¼ĮÖŠ£¬·ÖÉ¢ÖŹµÄÖ±¾¶ŌŚ1nm”«100nmÖ®¼ä£¬ŌņøĆ»ģŗĻĪļŹōÓŚ½ŗĢ壬ĖłŅŌĖłµĆ»ģŗĻĪļæÉÄܾßÓŠµÄŠŌÖŹŹĒ½ŗĢåµÄŠŌÖŹ£¬½ŗĢåµÄ·ÖÉ¢ÖŹĪ¢Į£½Ļ“󣬲»ÄÜĶعż°ėĶøĤ£¬µ«ÄÜĶø¹żĀĖÖ½½ŗĢ嶼ÄܲśÉś¶”“ļ¶ūŠ§Ó¦£¬¹Ź“š°øĪŖ£ŗB£»
£Ø2£©¢Ł½ŗĢåŗĶČÜŅŗµÄĒų±šŹĒ£ŗ½ŗĢå¾ßÓŠ¶”“ļ¶ūŠ§Ó¦£¬¶ųČÜŅŗ²»¾ß±ø£¬æÉŅŌ¾Ż“ĖĄ“¼ų±š¶žÕߣ¬¹Ź“š°øĪŖ£ŗČĆŅ»Źųæɼū¹ā·Ö±šÕÕÉäĮ½ĘæĪŽÉ«ŅŗĢ壬æɼūµ½Ņ»Ģõ¹āĮĮĶØĀ·µÄĪŖµķ·Ū½ŗĢ壻
¢Śµķ·ŪĖ®½āÉś³ÉĘĻĢŃĢĒ£¬½ŗĮ£²»ÄÜĶø¹ż°ėĶøĤ£¬ĢŃĢĒŹĒŠ”·Ö×Ó£¬ÄÜĶø¹ż£¬æÉÓĆÉųĪöĢį“æ½ŗĢ壬¹Ź“š°øĪŖ£ŗĀóŃæĢĒ£»ÉųĪö£®
µćĘĄ ±¾Ģāæ¼²é½ŗĢåµÄĢŲŠŌŅŌ¼°ČÜŅŗŗĶ½ŗĢåµÄ¼ų±š”¢Ģį“æÖŖŹ¶£¬ÄŃ¶Č½ĻŠ”£¬Ö¼ŌŚæ¼²éѧɜ¶Ō»ł“”ÖŖŹ¶µÄŹ¶¼Ē£¬×¢Ņā»ł“”ÖŖŹ¶µÄ»żĄŪÕĘĪÕ£®


 ŠĀ·Ē·²½Ģøسå“Ģ100·ÖĻµĮŠ“š°ø
ŠĀ·Ē·²½Ģøسå“Ģ100·ÖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
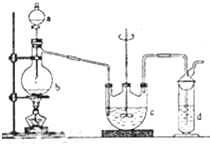 Įņ“śĮņĖįÄĘ£ØNa2S2O3£©Ė×Ćū“óĖÕ“ņ£¬æÉÓĆ×ö·ÖĪöŹŌ¼Į£®ĖüŅ×ČÜÓŚĖ®£¬ÄŃČÜÓŚ¾Ę¾«£¬ŹÜČČ”¢ÓöĖįŅ×·Ö½ā£®¹¤ŅµÉĻæÉÓĆĮņ»Æ¼ī·ØÖʱø£¬·“Ó¦ŌĄķ£ŗ
Įņ“śĮņĖįÄĘ£ØNa2S2O3£©Ė×Ćū“óĖÕ“ņ£¬æÉÓĆ×ö·ÖĪöŹŌ¼Į£®ĖüŅ×ČÜÓŚĖ®£¬ÄŃČÜÓŚ¾Ę¾«£¬ŹÜČČ”¢ÓöĖįŅ×·Ö½ā£®¹¤ŅµÉĻæÉÓĆĮņ»Æ¼ī·ØÖʱø£¬·“Ó¦ŌĄķ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ±ź×¼×“æöĻĀ£¬11.2LHClŗĶ11.2LNH3³ä·Ö»ģŗĻŗóŗ¬ÓŠµÄ·Ö×ÓŹżĪŖNA | |
| B£® | 0.1mol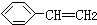 ÖŠŗ¬ÓŠĢ¼Ģ¼Ė«¼üµÄŹżÄæĪŖ0.4NA ÖŠŗ¬ÓŠĢ¼Ģ¼Ė«¼üµÄŹżÄæĪŖ0.4NA | |
| C£® | ³£ĪĀ³£Ń¹ĻĀ£¬1molNO2ÓėĖ®·“Ó¦ŗó£¬ČÜŅŗÖŠNO3-µÄŹżÄæĪŖNA | |
| D£® | 1molMgÓė×ćĮææÕĘų·“Ӧɜ³ÉMgOŗĶMg3N2£¬Ź§Č„µÄµē×ÓŹżĪŖ2NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
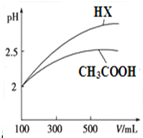 ŅŃÖŖ25”ꏱ²æ·ÖČõµē½āÖŹµÄµēĄėĘ½ŗā³£ŹżŹż¾ŻČē±ķĖłŹ¾£ŗ
ŅŃÖŖ25”ꏱ²æ·ÖČõµē½āÖŹµÄµēĄėĘ½ŗā³£ŹżŹż¾ŻČē±ķĖłŹ¾£ŗ| »ÆѧŹ½ | CH3COOH | H2CO3 | HClO | |
| µēĄėĘ½ŗā³£Źż | Ka=1.8”Į10-5 | Ka1=4.3”Į10-7 | Ka=5.6”Į10-11 | Ka=3.0”Į10-8 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
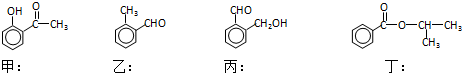
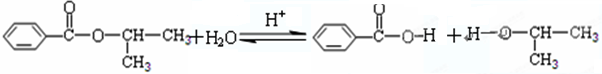 £»
£»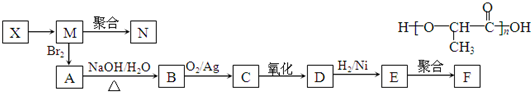
 £®
£®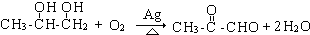 £»
£» £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | NH4+”¢Br-”¢CO32- | B£® | NH4+”¢I-”¢SO32- | C£® | Fe2+”¢I-”¢SO32- | D£® | Fe2+”¢Br-”¢CO32- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com