
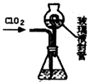
 �������ȣ�C1O2����Ŀǰ�����Ϲ��ϵĵ��Ĵ���Ч����������������һ�ֻ���ɫ�����壬������ˮ��ʵ���ҿ���Fa-14Cl�����ᡢNaCl02���������ƣ�Ϊԭ���Ʊ�C1O2���������£�
�������ȣ�C1O2����Ŀǰ�����Ϲ��ϵĵ��Ĵ���Ч����������������һ�ֻ���ɫ�����壬������ˮ��ʵ���ҿ���Fa-14Cl�����ᡢNaCl02���������ƣ�Ϊԭ���Ʊ�C1O2���������£�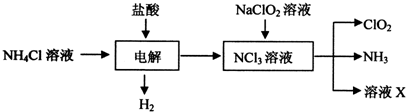
���� ��1������Ȼ�狀���������Һʱ����������ͼ֪������������NCl3��
��2������Ϣ��֪��ClO2������ˮ�����Բ�������ˮ��Һ���գ�����Ϊ�������壮�ݴ��жϣ�
��3������ˮ�ٴ����ղ���Ķ����������壬��ʹ��ƿ����ѹǿ��ȣ�
������Ŀ��Ϣ��֪��ClO2ͨ����ƿ�����Ե⻯����Һ��Ӧ������I-ΪI2����������ԭΪCl-��ͬʱ����ˮ��
����Һ����ɫǡ�ñ�Ϊ��ɫ���Ұ�����ڲ���ɫ��˵���ζ����յ㣻
�ܸ��ݹ�ϵʽ2ClO2��5I2��10Na2S2O3����n��ClO2�����ٸ���m=nM����m��ClO2����
����ʢ����������Ʊ���Һ�ĵζ���δ��ϴ��ϡ�ͱ���Һ���������ı���Һ������������
��� �⣺��1������Ȼ�狀���������Һʱ����������ͼ֪������������NCl3�����ⷴӦ����ʽΪ��NH4Cl+2HCl$\frac{\underline{\;ͨ��\;}}{\;}$3H2��+NCl3��
�ʴ�Ϊ��NH4Cl+2HCl$\frac{\underline{\;ͨ��\;}}{\;}$3H2��+NCl3��
��2��A��ClO2������ˮ���������ñ���ʳ��ˮ���հ�������A����
B����ʯ�Ҳ������հ�������B����
C��Ũ����������հ������Ҳ�Ӱ��ClO2����C��ȷ��
D��ClO2������ˮ����������ˮ���հ�������D����
�ʴ�Ϊ��C��
��3����װ���в���Һ��ܵ������ǣ���ˮ�ٴ����ղ���Ķ����������壬��ʹ��ƿ����ѹǿ��ȣ�
�ʴ�Ϊ����ˮ�ٴ����ղ���Ķ����������壬��ʹ��ƿ����ѹǿ��ȣ�
������Ŀ��Ϣ��֪��ClO2ͨ����ƿ�����Ե⻯����Һ��Ӧ������I-ΪI2����������ԭΪCl-��ͬʱ����ˮ����Ӧ���ӷ���ʽΪ2ClO2+10I-+8H+=2Cl-+5I2+4H2O��
�ʴ�Ϊ��2ClO2+10I-+8H+=2Cl-+5I2+4H2O��
�۵������һ��ʱ�����������Һʱ����Һ����ɫǡ�ñ�Ϊ��ɫ���Ұ�����ڲ���ɫ��˵���ζ����յ㣬
�ʴ�Ϊ����Һ����ɫǡ�ñ�Ϊ��ɫ���Ұ�����ڲ���ɫ��
��VmLNa2S2O3��Һ����Na2S2O3���ʵ���ΪV•10-3 L��cmol/L=c•V•10-3 mol����
���ݹ�ϵʽ��2ClO2��5I2��10Na2S2O3��
2 10
n��ClO2�� c•V•10-3 mol
����n��ClO2��=$\frac{1}{5}$c•V•10-3 mol��
����m��ClO2��=$\frac{1}{5}$c•V•10-3 mol��67.5g/mol=1.35cv��10-2g��
�ʴ�Ϊ��1.35cv��10-2��
����ʢ����������Ʊ���Һ�ĵζ���δ��ϴ��ϡ�ͱ���Һ���������ı���Һ������ⶨ���ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
���� ���⿼���Ķ���Ŀ��ȡ��Ϣ������������ԭ��Ӧ�ζ���Ӧ�á��Թ������̼�װ�����������֪ʶ����Ŀ�Ѷ��еȣ�Ҫ��ѧ��Ҫ����ʵ��ʵ�����֪ʶ�����Ӧ����Ϣ��������������ע�����֪ʶ��ȫ�����գ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������������ѧ�߶��ϵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�ʻ���(COS)����Ϊһ����ʳѬ����,�ܷ�ֹijЩ ���桢�߳�������Σ�����ں����ܱ�������,��CO��H2S��ϼ��Ȳ��ﵽ����ƽ��:CO(g)+H2S(g)
���桢�߳�������Σ�����ں����ܱ�������,��CO��H2S��ϼ��Ȳ��ﵽ����ƽ��:CO(g)+H2S(g) COS(g)+H2(g) K=0.1��ӦǰCO����
COS(g)+H2(g) K=0.1��ӦǰCO���� ����Ϊ10 mol,ƽ���CO���ʵ���Ϊ8 mol������˵����ȷ����
����Ϊ10 mol,ƽ���CO���ʵ���Ϊ8 mol������˵����ȷ����
A�������¶�,H2SŨ������,�����÷�Ӧ�����ȷ�Ӧ
B��ͨ��CO��,����Ӧ����������
C��CO��ƽ��ת����Ϊ80%
D����ӦǰH2S���ʵ���Ϊ7 mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ͭʱ�������Դ������������ͭ���Դ�ĸ������� | |
| B�� | ��ˮ�в���ͨ��CO2������CO2�����ӣ�$\frac{c��O{H}^{-}��}{c��N{H}_{3}•{H}_{2}O��}$�������� | |
| C�� | 3C��s��+CaO��s��=CaC2��s��+CO��g���ڳ����²����Է����У�˵���÷�Ӧ�ġ�H��0 | |
| D�� | �ϳɰ�ʱ�������������������¶ȣ���Ӧ����v��H2����������ƽ��ת���ʾ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2NA��HCl������44.8 L H2��Cl2�Ļ������������ԭ����Ŀ��Ϊ4NA | |
| B�� | 32gCu������Ũ��ϡ����ֱ�ԭΪNO2��NO��Ũ��ϡ����õ��ĵ�������ΪNA | |
| C�� | ���ʵ���Ũ�Ⱦ�Ϊ1mol/L��NaCl��MgCl2�����Һ�У�����Cl-����ĿΪ3NA | |
| D�� | 1molD3${\;}_{\;}^{18}$O+������D����${\;}_{1}^{2}$H���к��е�������Ϊ10NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢۢ� | B�� | �ۢݢޢ� | C�� | �٢ۢܢ� | D�� | �ڢۢޢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com