
ij����С���ͬѧ��ʵ������п��Ũ���ᷴӦ��ʵ��ʱ����ͬѧ��Ϊ�����������Ƕ���������ͬѧ��Ϊ���������������⣬�����ܲ���������Ϊ����֤�ס�����ͬѧ���ж��Ƿ���ȷ����ͬѧ�������ͼ��ʾ��ʵ��װ��(п��Ũ���Ṳ��ʱ����������ΪX���Ҹ÷�Ӧװ������ȥ)��

�Իش�
(1)����ʵ�������ɶ�����������Ļ�ѧ��Ӧ����ʽΪ_________________________
________________________________________________________________________��
(2)��ͬѧ��Ϊ�����ܲ���������������___________________________________
________________________________________________________________________��
(3)��ͬѧ�ڰ�װ��װ�úز����ٵ�һ��������
________________________________________________________________________��
(4)B�м�����Լ���________����������___________________________________
________________________________________________________________________��
(5)����֤������X�к���������ʵ�������ǣ�C��________________________��D��________________________�������ȥװ��B���Ƿ��ܸ���D�е������ж�����X�к���������____________��������________________________________________________
________________________________________________________________________��
(1)Zn��2H2SO4(Ũ)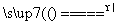 ZnSO4��SO2����2H2O
ZnSO4��SO2����2H2O
(2)��п��Ũ����ķ�Ӧ�У�H2SO4�����ģ�����ˮ���ɣ�c(H2SO4)��С����Ũ������ϡ���ᣬп��ϡ���ᷴӦ��������
(3)���װ��������
(4)Ũ���ᡡ����ˮ����
(5)��ɫ����ͭ��ĩ��ɺ�ɫ����ˮ����ͭ��ĩ�����ɫ�����ܡ��������ͨ������KMnO4��Һʱ�����ˮ���������ź���ʵ����H2�ļ���
����������ʱӦ������ĿҪ��������������Ե�˼·�����Ȼ���ٰ�ÿ��������á�Ϊȷ��SO2����������A�м���Ʒ����Һ������������KMnO4��Һ�����ȥ��Ϊȷ��H2����������H2��ԭCuO������������H2O������������B�������壬��������˼·�������ˡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)����֬A�Ľṹ��ʽ
 �����������ܷ����Ļ�ѧ��Ӧ��
�����������ܷ����Ļ�ѧ��Ӧ��
�ٴӱ����Կ������ܷ���________��Ӧ��������������Ӧ�Ļ�ѧ����ʽΪ
________________________________________________________________��
�ڴ��������ʿ������ܷ���________��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ
________________________________________________________________��
(2)�о�Ӳ���͵����ʺ���;____________________________________
________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y��Z�ֱ������ֳ����Ľ�����ǽ������ʣ� M��N��R�dz������������������һ�־��и��۵㣬���������·�Ӧ������δ���������δ��ƽ������X + Z �� N����M+X �� N���� M+Y�� R+X ����X�Ƿǽ����������Y���ʵ�Ԫ�������ڱ��е�λ����
A���ڶ����ڢ�A�� B���������ڢ�A��
C���ڶ����ڢ�A�� D���������ڢ�A��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ˮ�ȷ��Ʊ�ֱ��Ϊ1~100 nm�Ŀ���Y�����������Ӧԭ��Ϊ��
3Fe2+ + 2S2O32- + O2 + aOH��== Y+ S4O62- + 2H2O������˵���в���ȷ����
A��a��4
B����Y���ȷ�ɢ��ˮ���γɵ���ϵ���ж����ЧӦ
C��ÿ��3 mol Fe2+ �μӷ�Ӧ����Ӧ��ת�Ƶĵ�������Ϊ5 mol
D��S2O32-�ǻ�ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����з�Ӧ�У�����ȱ����������ֱ������Ե���(����)
A��FeO��HNO3 B��Al(OH)3��HNO3
C��CuO��HNO3 D��CaCO3��HNO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и��л����ϵͳ������ȷ���ǣ� ��
A��2��3,3���������� B�� 2���һ�����
C��2������2����ϩ D��2,2��������1,3������ϩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ������ij�л�����ȫȼ�պ�ȼ�ղ���ͨ��������ʯ��ˮ�������˿ɵó���10�ˣ���������Һʱ������ֻ����2.9�ˣ�����л��ﲻ������(���� )
A. �Ҷ��� B.��ϩ C.��ϩ D. ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����A��B��C��D��E��Fԭ�������������������Ԫ�أ�����λ��Ԫ�����ڱ���ǰ�����ڣ�AԪ��ԭ�ӵĺ������������������������ȣ�BԪ��ԭ�Ӻ�����3���ܼ����е��ӣ���ÿ���ܼ��ϵĵ�������ͬ��DԪ��ԭ�Ӻ�����8���˶�״̬��ͬ�ĵ��ӣ�EԪ����FԪ�ش���ͬһ�������ڵ��壬���ǵ�ԭ���������3,��EԪ�صĻ�̬ԭ����4��δ�ɶԵ��ӡ���ش��������⣺
(1) EԪ�ػ�̬ԭ�ӵĺ���۲�����Ų�ʽΪ_________
(2)��Ԫ�ط��ű�ʾB��C��D����Ԫ�صĵ�һ�������ɵ͵��ߵ�����_________��
(3) AԪ����B��CԪ�ؿ��γɻ�����B2A4��C2A4��ֱ�ṹʽΪ_________ _________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A�ǻ�ѧʵ������������л���ܽ�����ͼ��ʾ�Ķ��ַ�Ӧ��
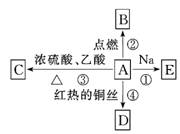 |
��1��A������____ ___��C�ķ���ʽΪ___ _____��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ��
��Ӧ��___________ ___________________________��
��Ӧ��_____________________ _________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com