
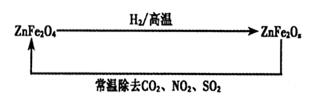
 Al2O3£«2Fe £Ø2£©4Fe(s)+3O2 (g)£½2Fe2O3(s) ”÷H£½£1648.7kJ/mol
Al2O3£«2Fe £Ø2£©4Fe(s)+3O2 (g)£½2Fe2O3(s) ”÷H£½£1648.7kJ/mol Al2O3£«2Fe£»
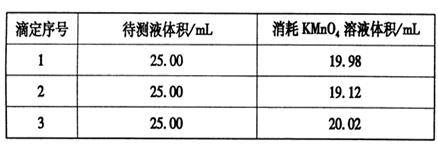
Al2O3£«2Fe£»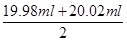 £½20.00ml£¬ĖłŅŌøł¾Ż·½³ĢŹ½5Fe2£«£«MnO4££«8H£«£½5Fe3£«£«Mn2£«£«4H2OæÉÖŖ£¬FeSO4ČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ
£½20.00ml£¬ĖłŅŌøł¾Ż·½³ĢŹ½5Fe2£«£«MnO4££«8H£«£½5Fe3£«£«Mn2£«£«4H2OæÉÖŖ£¬FeSO4ČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ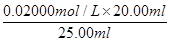 ”Į5£½0.080 00 mol”¤L-1”£
”Į5£½0.080 00 mol”¤L-1”£

 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā

 C¶ŌÓ¦µÄ»Æѧ·½³ĢŹ½ ”£
C¶ŌÓ¦µÄ»Æѧ·½³ĢŹ½ ”£| A£®µĪ¶Ø¹ÜŌŚŹ¢×°øßĆĢĖį¼ŲĒ°Ī“ČóĻ“ |
| B£®µĪ¶Ø¹ż³ĢÖŠ£¬×¶ŠĪĘæÕšµ“µÄĢ«¾ēĮŅ£¬ŅŌÖĀ²æ·ÖŅŗĢ彦³ö |
| C£®µĪ¶ØĒ°¶ĮŹżÕżČ·£¬µĪ¶ØÖÕµćŹ±ø©ŹÓ¶ĮŹż |
| D£®µĪ¶ØĒ°¶ĮŹżÕżČ·£¬µĪ¶ØÖÕµćŹ±ŃöŹÓ¶ĮŹż |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
| ŹµŃé²½Öč | ĻÖĻóÓė½įĀŪ |
| ²½Öč1:ĻņŹŌ¹ÜÖŠ¼ÓČėÉŁĮæ¹ĢĢå²śĪļ,ŌŁ¼ÓČė×ćĮæ ,³ä·ÖÕńµ“ | ČōČÜŅŗŃÕÉ«Ć÷ĻŌøıä,ĒŅÓŠ Éś³É,ŌņÖ¤Ć÷ÓŠĢśµ„ÖŹ“ęŌŚ |
| ²½Öč2:½«²½Öč1ÖŠµĆµ½µÄ×ĒŅŗ¹żĀĖ,²¢ÓĆÕōĮóĖ®Ļ“µÓÖĮĻ“µÓŅŗĪŽÉ« | |
| ²½Öč3:Č”²½Öč2µĆµ½µÄÉŁĮæ¹ĢĢåÓŚŹŌ¹ÜÖŠ,µĪ¼Ó | |
 +3Mn
+3Mn +24H+
+24H+ 5Fe3+ +10CO2”ü+3Mn2++12H2OŹµŃé·½°øÉč¼ĘĪŖ:
5Fe3+ +10CO2”ü+3Mn2++12H2OŹµŃé·½°øÉč¼ĘĪŖ: )= ;FeC2O4µÄÖŹĮæ·ÖŹżĪŖ (¾«Č·µ½0.01%)”£
)= ;FeC2O4µÄÖŹĮæ·ÖŹżĪŖ (¾«Č·µ½0.01%)”£ ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®19.2 g | B£®11.52 g | C£®5.76 g | D£®9.6 g |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

 CeO2”¤8OH + 8_____”ü£»CeO2”¤8OH
CeO2”¤8OH + 8_____”ü£»CeO2”¤8OH CeO2+ 4H2O”ü+2O2”ü”£
CeO2+ 4H2Oӟ+2O2ӟӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
 Ģį¹©ÄÜĮ攣ĻĀĮŠÓŠ¹ŲŠšŹöÕżČ·µÄŹĒ( )
Ģį¹©ÄÜĮ攣ĻĀĮŠÓŠ¹ŲŠšŹöÕżČ·µÄŹĒ( )| A£®øĆČ¼ĮĻĀĢÉ«»·±££¬ŌŚČ¼ÉÕ¹ż³ĢÖŠ²»»įŌģ³ÉČĪŗĪ»·¾³ĪŪČ¾ |
| B£®øĆ·“Ó¦ÖŠN2O4ŹĒŃõ»Æ¼Į£¬Ę«¶ž¼×ėĀŹĒ»¹Ō¼Į |
| C£®N2¼ČŹĒŃõ»Æ²śĪļÓÖŹĒ»¹Ō²śĪļ£¬CO2¼Č²»ŹĒŃõ»Æ²śĪļŅ²²»ŹĒ»¹Ō²śĪļ |
| D£®ĆæÓŠ0.6 mol N2Éś³É£¬×ŖŅʵē×ÓŹżÄæĪŖ2.4NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
 2 K2S + K2SO3 + 3 H2O
2 K2S + K2SO3 + 3 H2O| A£®a = 2b | B£®x = 2 | C£®n = 0.48 | D£®b = 0.02 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com