
| 20”ę | 60”ę | 100”ę | |
| Na2SO4 | 19.5 | 45.3 | 42.5 |
| Na2Cr2O7[Ą“Ō“£ŗ] | 183 | 269 | 415 |
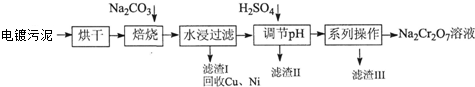
·ÖĪö £Ø1£©ŅŌŗ¬ÓŠCr£ØOH£©3”¢Al2O3”¢ZnO”¢CuO”¢NiOµČĪļÖŹµÄµē¶ĘĪŪÄąĪŖŌĮĻ£¬ŗęøÉŗó¼ÓČėĢ¼ĖįÄĘŗĶŃõĘųøßĪĀ×ĘÉÕ·¢Éś·“Ó¦4Cr£ØOH£©3+4Na2CO3+3O2$\frac{\underline{\;øßĪĀ\;}}{\;}$4Na2CrO4+6H2O+4CO2£¬Al2O3+Na2CO3$\frac{\underline{\;øßĪĀ\;}}{\;}$Na2AlO3+CO2”ü£¬ZnO+Na2CO3$\frac{\underline{\;øßĪĀ\;}}{\;}$Na2ZnO2+CO2”ü£¬Ė®½žŗó¹żĀĖµĆµ½ĀĖŌüCuO”¢NiO£¬ĀĖŅŗĪŖNa2CrO4£¬NaAlO2”¢Na2ZnO2µČ£¬µ÷½ŚČÜŅŗPH³ĮµķZnO22-Ąė×ÓŗĶĘ«ĀĮĖįøłĄė×Ó£¬¹żĀĖµĆµ½ĀĖŅŗNa2CrO4£¬ĀĖŌü¢ņµÄÖ÷ŅŖ³É·ÖÓŠZn£ØOH£©2”¢Al£ØOH£©3£¬ĀĖŅŗNa2CrO4¼ÓČėĮņĖįĖį»ÆÉś³ÉÖŲøõĖįÄĘČÜŅŗ£¬ĶعżĢį“æµĆµ½ÖŲøõĖįÄĘ£¬¾Ż“Ė·ÖĪö£»
£Ø2£©øł¾ŻÅŠ¶ĻĘ½ŗāדĢ¬µÄ·½·Ø£ŗVÕż=VÄę£¬»ņø÷×é·ÖµÄÅØ¶Č±£³Ö²»±äŌņĖµĆ÷ŅŃ“ļĘ½ŗā£¬
A£®ČÜŅŗµÄpHÖµ±£³Ö²»±ä£¬ĖµĆ÷ĒāĄė×ÓÅØ¶Č²»±ä£¬ÄÜÅŠ¶ĻĘ½ŗā£»
B£®¦ĶÕż£ØCr2O72-£©=$\frac{1}{2}$¦ĶÕż£ØCrO42-£©=2¦ĶÄę£ØCrO42-£©£¬VÕż”ŁVÄę²»ÄÜÅŠ¶ĻĘ½ŗā£»
C£®Cr2O72-ŗĶCrO42-µÄÅضČĻąĶ¬Č”¾öÓŚĘšŹ¼ÅضČŗĶ×Ŗ»Æ£¬²»ÄÜÅŠ¶ĻĘ½ŗā£»
D£®ČÜŅŗµÄŃÕÉ«²»±ä£¬ĪŖĢŲÕ÷¶Ø£¬ÄÜÅŠ¶ĻĘ½ŗā£»
£Ø3£©Ėį»ÆŹ±·¢ÉśµÄ·“Ó¦ĪŖ£ŗ2CrO42-+2H+?Cr2O72-+H2O£¬-
øł¾ŻŹŲŗćĮŠ¹ŲĻµŹ½£ŗ2Cr”«2CrO42-”«Cr2O72-£¬ĮŠŹ½¼ĘĖćµĆn£ØCr2O72-£©ŗĶn£ØCrO42-£©Ź£Óą£»
½ų¶ųĒó³öĖį»ÆŗóĖłµĆČÜŅŗÖŠc£ØCr2O72-£©
¢Śøł¾Ż·“Ó¦ 2CrO42-+2H+ØT?Cr2O72-+H2O
¼°Ę½ŗā³£ŹżKØT2”Į1013£¬½ųŠŠ¼ĘĖć£»
£Ø4£©ĻņNa2Cr2O7ÓėH2SO4»ģŗĻŅŗÖŠ¼ÓČėH2O2Éś³ÉCrO5£¬Na2SO4ŗĶH2O£¬¾Ż“ĖŹéŠ“·½³ĢŹ½£®
½ā“š ½ā£ŗŅŌŗ¬ÓŠCr£ØOH£©3”¢Al2O3”¢ZnO”¢CuO”¢NiOµČĪļÖŹµÄµē¶ĘĪŪÄąĪŖŌĮĻ£¬ŗęøÉŗó¼ÓČėĢ¼ĖįÄĘŗĶŃõĘųøßĪĀ×ĘÉÕ·¢Éś·“Ó¦4Cr£ØOH£©3+4Na2CO3+3O2$\frac{\underline{\;øßĪĀ\;}}{\;}$4Na2CrO4+6H2O+4CO2£¬Al2O3+Na2CO3$\frac{\underline{\;øßĪĀ\;}}{\;}$Na2AlO3+CO2”ü£¬ZnO+Na2CO3$\frac{\underline{\;øßĪĀ\;}}{\;}$Na2ZnO2+CO2”ü£¬Ė®½žŗó¹żĀĖµĆµ½ĀĖŌüCuO”¢NiO£¬ĀĖŅŗĪŖNa2CrO4£¬NaAlO2”¢Na2ZnO2µČ£¬µ÷½ŚČÜŅŗPH³ĮµķZnO22-Ąė×ÓŗĶĘ«ĀĮĖįøłĄė×Ó£¬¹żĀĖµĆµ½ĀĖŅŗNa2CrO4£¬ĀĖŌü¢ņµÄÖ÷ŅŖ³É·ÖÓŠZn£ØOH£©2”¢Al£ØOH£©3£¬ĀĖŅŗNa2CrO4¼ÓČėĮņĖįĖį»ÆÉś³ÉÖŲøõĖįÄĘČÜŅŗ£¬ĶعżĢį“æµĆµ½ÖŲøõĖįÄĘ£¬ŌņĀĖŌü¢óµÄÖ÷ŅŖ³É·ŻĪŖNa2SO4•10H2O£¬
£Ø1£©øł¾ŻŅŌÉĻ·ÖĪö£¬±ŗÉÕ¹ż³ĢÖŠÉś³ÉNa2CrO4µÄ»Æѧ·½³ĢŹ½ĪŖ4Cr£ØOH£©3+4Na2CO3+3O2$\frac{\underline{\;øßĪĀ\;}}{\;}$4Na2CrO4+6H2O+4CO2£¬Al2O3+Na2CO3$\frac{\underline{\;øßĪĀ\;}}{\;}$Na2AlO3+CO2”ü£¬ZnO+Na2CO3$\frac{\underline{\;øßĪĀ\;}}{\;}$Na2ZnO2+CO2”ü£¬Ė®½žŗóČÜŅŗÖŠ³żNa2CrO4”¢NaAlO2Ķā»¹“ęŌŚµÄČÜÖŹÓŠNa2ZnO2£¬ĀĖŅŗNa2CrO4¼ÓČėĮņĖįĖį»ÆÉś³ÉÖŲøõĖįÄĘČÜŅŗ£¬ĶعżĢį“æµĆµ½ÖŲøõĖįÄĘ£¬ŌņĀĖŌü¢óµÄÖ÷ŅŖ³É·ŻĪŖNa2SO4•10H2O£»
¹Ź“š°øĪŖ£ŗ4Cr£ØOH£©3+4Na2CO3+3O2$\frac{\underline{\;øßĪĀ\;}}{\;}$4Na2CrO4+6H2O+4CO2£¬Al2O3+Na2CO3$\frac{\underline{\;øßĪĀ\;}}{\;}$Na2AlO3+CO2”ü£¬ZnO+Na2CO3$\frac{\underline{\;øßĪĀ\;}}{\;}$Na2ZnO2+CO2”ü£»Na2ZnO2£»Na2SO4•10H2O£»
£Ø2£©øł¾ŻÅŠ¶ĻĘ½ŗāדĢ¬µÄ·½·Ø£ŗVÕż=VÄę£¬»ņø÷×é·ÖµÄÅØ¶Č±£³Ö²»±äŌņĖµĆ÷ŅŃ“ļĘ½ŗā£¬
A£®ČÜŅŗµÄpHÖµ±£³Ö²»±ä£¬ĖµĆ÷ĒāĄė×ÓÅØ¶Č²»±ä£¬ÄÜÅŠ¶ĻĘ½ŗā£¬¹ŹAÕżČ·£»
B£®¦ĶÕż£ØCr2O72-£©=$\frac{1}{2}$¦ĶÕż£ØCrO42-£©=2¦ĶÄę£ØCrO42-£©£¬VÕż”ŁVÄę²»ÄÜÅŠ¶ĻĘ½ŗā£¬¹ŹB“ķĪó£»
C£®Cr2O72-ŗĶCrO42-µÄÅضČĻąĶ¬Č”¾öÓŚĘšŹ¼ÅضČŗĶ×Ŗ»Æ£¬²»ÄÜÅŠ¶ĻĘ½ŗā£¬¹ŹC“ķĪó£»
D£®ČÜŅŗµÄŃÕÉ«²»±ä£¬ĪŖĢŲÕ÷¶Ø£¬ÄÜÅŠ¶ĻĘ½ŗā£¬¹ŹDÕżČ·£»
¹Ź“š°øĪŖ£ŗAD£»
£Ø2£©Ėį»ÆŹ±·¢ÉśµÄ·“Ó¦ĪŖ£ŗ2CrO42-+2H+?Cr2O72-+H2O£¬Čō1LĖį»ÆŗóĖłµĆČÜŅŗÖŠŗ¬øõŌŖĖŲµÄÖŹĮæĪŖ23.4g£¬CrO42-ÓŠ$\frac{8}{9}$×Ŗ»ÆĪŖCr2O72-£¬ĖµĆ÷øõŌŖĖŲ$\frac{8}{9}$×Ŗ»ÆĪŖCr2O72-
øł¾ŻŹŲŗćĮŠ¹ŲĻµŹ½£ŗ2Cr”«2CrO42-”«Cr2O72-
2 1
$\frac{23.4”Į\frac{8}{9}}{52}$ n£ØCr2O72-£©
µĆn£ØCr2O72-£©=0.2mol£»n£ØCrO42-£©Ź£Óą=0.05mol£»
Ōņ¢ŁĖį»ÆŗóĖłµĆČÜŅŗÖŠc£ØCr2O72-£©=$\frac{n}{V}$=0.2mol•L-1£»¹Ź“š°øĪŖ£ŗ0.2mol•L-1£»
¢ŚÉčH+µÄĪļÖŹµÄĮæÅضČĪŖamol/L£¬
2CrO42-+2H+ØT?Cr2O72-+H2O
Ę½ŗā£Ømol/L£© 0.05 a 0.2
Ę½ŗā³£ŹżK=$\frac{0.2}{0.05{\;}^{2}”Įa{\;}^{2}}$ØT2”Į1013£¬
Ōņa=4”Į10-7mol£¬PH=7-2lg2=6.4£¬
¹Ź“š°øĪŖ£ŗ6.4£»
£Ø4£©ĻņNa2Cr2O7ÓėH2SO4»ģŗĻŅŗÖŠ¼ÓČėH2O2Éś³ÉCrO5£¬Na2SO4ŗĶH2O£¬Ōņ·½³ĢŹ½ĪŖ£ŗNa2Cr2O7+4H2O2+H2SO4ØT2CrO5+Na2SO4+5H2O£¬¹Ź“š°øĪŖ£»Na2Cr2O7+4H2O2+H2SO4ØT2CrO5+Na2SO4+5H2O£®
µćĘĄ ±¾Ģāæ¼²éĮĖĪļÖŹµÄ·ÖĄėĢį“æĮ÷³Ģ£¬Ö÷ŅŖæ¼²éĮĖ²Ł×÷Į÷³Ģ·ÖĪö”¢Ę½ŗāדĢ¬µÄÅŠ¶Ļ”¢ČܶȻż³£ŹżµÄ¼ĘĖć”¢·½³ĢŹ½µÄŹéŠ“µČ£¬ĢāÄæÄѶČÖŠµČ£¬²ąÖŲÓŚæ¼²éѧɜ·ÖĪöĪŹĢā”¢½ā¾öĪŹĢāÄÜĮ¦ŗĶ¼ĘĖćÄÜĮ¦£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A×é | B×é |
| ¢ń£®H-F¼üµÄ¼üÄÜ“óÓŚH-O¼üµÄ¼üÄÜ | ¢ŁHFµÄ·Šµć±ČH2OµĶ |
| ¢ņ£®H-H”FĒā¼üµÄ¼üÄÜ“óÓŚO-H”OĒā¼üµÄ¼üÄÜ | ¢ŚHF±ČH2OĪČ¶Ø |
| ¢ó£®HF·Ö×Ó¼äÄÜŠĪ³ÉµÄĒā¼üøöŹż±ČH2O·Ö×ÓÉŁ | ¢ŪHFµÄ·Šµć±ČH2Oøß |
| ¢ō£®HF·Ö×ÓÓėĖ®·Ö×ÓæÉŠĪ³ÉĒā¼ü | ¢ÜHF¼«Ņ×ČÜÓŚĖ® |
| A£® | I¢Ś | B£® | ¢ņ¢Ū | C£® | ¢ó¢Ł | D£® | ¢ó¢Ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
 ijĢžAŹĒÓŠ»ś»Æѧ¹¤ŅµµÄ»ł±¾ŌĮĻ£¬Ęä²śĮææÉŅŌÓĆĄ“ŗāĮæŅ»øö¹ś¼ŅµÄŹÆÓĶ»Æ¹¤·¢Õ¹Ė®Ę½£¬A»¹ŹĒŅ»ÖÖÖ²ĪļÉś³¤µ÷½Ś¼Į£¬AæÉ·¢ÉśČēĶ¼ĖłŹ¾µÄ Ņ»ĻµĮŠ»Æѧ·“Ó¦£¬ĘäÖŠ¢Ł¢Ś¢ŪŹōÓŚĶ¬ÖÖ·“Ó¦ĄąŠĶ£®øł¾ŻĶ¼»Ų“šĻĀĮŠĪŹĢā£ŗ
ijĢžAŹĒÓŠ»ś»Æѧ¹¤ŅµµÄ»ł±¾ŌĮĻ£¬Ęä²śĮææÉŅŌÓĆĄ“ŗāĮæŅ»øö¹ś¼ŅµÄŹÆÓĶ»Æ¹¤·¢Õ¹Ė®Ę½£¬A»¹ŹĒŅ»ÖÖÖ²ĪļÉś³¤µ÷½Ś¼Į£¬AæÉ·¢ÉśČēĶ¼ĖłŹ¾µÄ Ņ»ĻµĮŠ»Æѧ·“Ó¦£¬ĘäÖŠ¢Ł¢Ś¢ŪŹōÓŚĶ¬ÖÖ·“Ó¦ĄąŠĶ£®øł¾ŻĶ¼»Ų“šĻĀĮŠĪŹĢā£ŗ £¬·“Ó¦ĄąŠĶ¼Ó¾Ū·“Ó¦£®
£¬·“Ó¦ĄąŠĶ¼Ó¾Ū·“Ó¦£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ĪĀ¶Č | K1 | K2 |
| 973K | 1.47 | 2.38 |
| 1173K | 2.15 | 1.67 |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Mg2+”¢NH${\;}_{4}^{+}$”¢OH-”¢SO${\;}_{4}^{2-}$ | B£® | Na+”¢H+”¢Cl-”¢HCO${\;}_{3}^{-}$ | ||
| C£® | Na+”¢K+”¢AlO${\;}_{2}^{-}$”¢CO${\;}_{3}^{2-}$ | D£® | H++”¢K+”¢MnO${\;}_{4}^{-}$”¢SO${\;}_{3}^{2-}$ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | O2-µÄ½į¹¹Ź¾ŅāĶ¼ĪŖ | |
| B£® | ijĪ¢Į£ŗĖĶāµē×ÓÅŲ¼ĪŖ2”¢8”¢8½į¹¹£¬ŌņøĆĪ¢Į£Ņ»¶ØŹĒė²Ō×Ó | |
| C£® | NH4+ÓėH3O+¾ßÓŠĻąĶ¬µÄÖŹ×ÓŹżŗĶµē×ÓŹż | |
| D£® | F-”¢Na+”¢Mg2+”¢Al3+ŹĒÓėHeŌ×Ó¾ßÓŠĻąĶ¬µē×Ó²ć½į¹¹µÄĄė×Ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | “ņæŖĘ”¾ĘĘæøĒŗóÓŠ“óĮæÅŻÄŅē³ö | |
| B£® | ŹµŃéŹŅÓĆÅű„ŗĶŹ³ŃĪĖ®µÄ·½·ØŹÕ¼ÆĀČĘų | |
| C£® | Čȵēæ¼īČ„ÓĶĪŪÄÜĮ¦øüĒæ | |
| D£® | ĻÄĢģ½«Ź³Ę·“¢²ŲŌŚ±łĻäÖŠ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
 |  |  |  |
| Ęū³µĪ²Ęų | ·ŁÉÕ·ĻĘśĖÜĮĻ | ¾ÓŹŅ×°äź | ×÷ÖĘĄä¼Į |
| A | B | C | D |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com