
���� ��1��ʵ��ʱӦ�ȳ���һ�������Ĺ��壬�ܽ�����Ƴ���Һ����ȡ����Һ����ƿ�У�Ȼ���ñ�Һ���еζ���
��2���ζ���0�̶����ϣ��ζ�ǰӦ���ڵ���̶Ȼ������µ�ijһ�̶ȣ�Ϊ��С�����첿��Ӧ����Һ�壬�����ݣ�
��3��ָʾ��Ϊ���ȣ���ɫ��ΧΪ3.1-4.4��
��4�����ݷ�Ӧ���ĵ����ᣬ����������ƣ���һ�������Ʒ�Ĵ��ȣ�
��5���ٲ���B�еļ�ʽ�ζ���ֻ������ˮϴδ����ʢ�ռ���Һ��ϴ������Һ��Һ��ϡ�ͣ������ĵ�������ƫС��
����ʽ�ζ���������ˮϴ�Ӻ�ֱ��װ���H2SO4��Һ���ᱻϡ�ͣ�������ƫ��
�۵ζ�ʱ����ƿҡ��̫���ң�������Һ�彦��������ҺƫС�������ĵ�������ƫС��
�ܵζ����յ�ʱ���ζ��ܼ��첿������Һ�Σ��������������ƫ��
����ʽ�ζ��ܶ���ʱ�ζ�ǰ���ӣ��ζ����ӣ�������������ƫС��
��� �⣺��1��ʵ��ʱӦ�ȳ���һ�������Ĺ��壬�ܽ�����Ƴ���Һ����ȡ����Һ����ƿ�У�Ȼ���ñ�Һ���еζ���
�ʴ�Ϊ��C��A��B��E��
��2�������ʵ���Ũ��ΪC mol/L�ı�H2SO4��Һװ����ʽ�ζ��ܣ�����Һ�浽��0���̶����£��ʴ�Ϊ����0���̶����£�
��3������ζ��ռ������ָʾ�����ζ����յ�������ǣ����������һ��H2SO4��Һ����ƿ����Һ�ɻ�ɫ��Ϊ��ɫ�����Ұ�����ڲ���ɫ��
�ʴ�Ϊ�����������һ��H2SO4��Һ����ƿ����Һ�ɻ�ɫ��Ϊ��ɫ�����Ұ�����ڲ���ɫ��
��4���ε����ĵ�����Ϊ��n�����ᣩ=cV=��V2-V1����10-3L��m mol/L�����ݷ�Ӧ���̿�֪��n��NaOH��=2n�����ᣩ=2m��V2-V1����10-3mol��
����ԭ����Ʒ���������Ƶ����ʵ���Ϊ��2m��V2-V1����10-3mol��$\frac{250mL}{25}$=2m��V2-V1����10-2mol������Ʒ���������Ƶ�����Ϊm��NaOH��=nM=80m��V2-V1����10-2g������ռ���Ʒ�Ĵ���Ϊ��$\frac{80c��{V}_{2}-{V}_{1}����1{0}^{-2}g}{mg}$��100%=$\frac{0.8c��{V}_{2}-{V}_{1}��}{m}$��100%��
�ʴ�Ϊ��$\frac{0.8c��{V}_{2}-{V}_{1}��}{m}$��100%��
��5���ٲ���B�еļ�ʽ�ζ���ֻ������ˮϴδ����ʢ�ռ���Һ��ϴ������Һ��Һ��ϡ�ͣ������ĵ�������ƫС�����Իᵼ�²ⶨ���ƫ�ͣ�
����ʽ�ζ���������ˮϴ�Ӻ�ֱ��װ���H2SO4��Һ���ᱻϡ�ͣ�������ƫ�����Իᵼ�²ⶨ���ƫ�ߣ�
�۵ζ�ʱ����ƿҡ��̫���ң�������Һ�彦��������ҺƫС�������ĵ�������ƫС�����Իᵼ�²ⶨ���ƫ�ͣ�
�ܵζ����յ�ʱ���ζ��ܼ��첿������Һ�Σ��������������ƫ�����Իᵼ�²ⶨ���ƫ�ߣ�
����ʽ�ζ��ܶ���ʱ�ζ�ǰ���ӣ��ζ����ӣ�������������ƫС�����Իᵼ�²ⶨ���ƫ�ͣ�
��ѡ�ڢܣ�
���� �����ۺϿ�������к͵ζ��������ڻ�ѧʵ����������Լ����ʵĺ����IJⶨ�����⣬��Ŀ�Ѷ��еȣ�������ѧϰ�а�����ػ���ʵ�鷽����ѧϰ��ע����ۣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
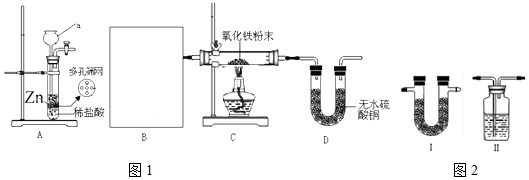
 ��ʵ�����ʱӦ�ò�ȡ�IJ�������ֹͣ���ȣ���ȴ�����£���ֹͣͨH2��
��ʵ�����ʱӦ�ò�ȡ�IJ�������ֹͣ���ȣ���ȴ�����£���ֹͣͨH2���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ɫ������Һ�У�Na+��SO42-��MnO4-��NO3- | |
| B�� | ʹ��ɫ��̪��Һ�ʺ�ɫ����Һ�У�Na+��Cu2+��SO42-��Cl- | |
| C�� | pH=1����Һ�У�K+��ClO-��S2-��Cl- | |
| D�� | �����Ե���Һ�У�Na+��K+��HCO3-��NO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �ζ����� | ����NaOH��Һ�����/mL | 0.100 0mol•L-1��������/mL | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
| ��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
| �ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
| ������ | 25.00 | 0.22 | 26.31 | 26.09 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ��� ���� | ������Һ��� V/mL | ����������Һ��� V/mL |
| 1 | 19.90 | 10.00 |
| 2 | 20.10 | 10.00 |
| 3 | 22.00 | 10.00 |
| 4 | 20.00 | 10.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �ζ����� | ������Һ �����mL�� | ������� | |
| �ζ�ǰ�Ŀ̶� ��mL�� | �ζ���Ŀ̶� ��mL�� | ||
| ��һ�� | 10.00 | 0.40 | 20.50 |
| �ڶ��� | 10.00 | 4.10 | 24.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��������һ����Ҫ���л�����ԭ�ϣ��Ʊ��������ԭ����95%�Ҵ���80%���ᣨ������ˮϡ��Ũ���ᣩ����ϸ���廯�Ʒ�ĩ�ͼ������Ƭ���÷�Ӧ��ԭ�����£�NaBr+H2SO4=NaHSO4+HBr
��������һ����Ҫ���л�����ԭ�ϣ��Ʊ��������ԭ����95%�Ҵ���80%���ᣨ������ˮϡ��Ũ���ᣩ����ϸ���廯�Ʒ�ĩ�ͼ������Ƭ���÷�Ӧ��ԭ�����£�NaBr+H2SO4=NaHSO4+HBr| ���� ���� | �Ҵ� | ������ | 1��2-�������� | ���� | Ũ���� |
| �ܶ�/g•cm-3 | 0.79 | 1.46 | 2.2 | 0.71 | 1.84 |
| �۵㣨�棩 | -130 | -119 | 9 | -116 | 10 |
| �е㣨�棩 | 78.5 | 38.4 | 132 | 34.6 | 338 |
| ��ˮ�е��ܽ�ȣ�g�� | ���� | 0.914 | 1 | 7.5 | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
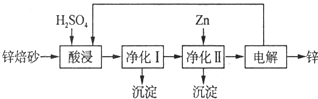
 ��
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com