
(12��)ijѧϰС��������ͼ��ʾװ�ý��С�����ˮ����Ӧ��������ʵ��(��ȥ�˼г�����)��
(1)Ϊ�˰�ȫ������E��ǰ������еIJ�����____________________________��
(2) B�з�����Ӧ�Ļ�ѧ����ʽ�� _______________��
(3)��֪�з�Ӧ��Cu2O+2H+Cu+Cu2++H2O������������鷴Ӧ��E�еĺ�ɫ�����г�Cu���Ƿ�Cu2O�����õ��Լ���________(�����)
a��ϡ���� b��ϡ���� c������ d��Ũ����
(4)��E�����ٽӶ���ʢ��ʯ�ҵĸ����(���γ�ΪF��G)�����ø�װ�òⶨˮ����ɡ�����Ӧ��ʢ��ҩƷ��E������������eg��F������������f g��ˮ��Ԫ�ص����ʵ���֮�ȿɱ�ʾΪ�����û���n(H)��n(O)= ________��_______������Ӧ��E�г�Cu�������һ�ֻ�ԭ����Cu2O����ñ�ֵ��___________ (ѡ�ƫ��ƫС������Ӱ�족)��
(5)ijѧ��Ϊ�˼��鷴Ӧ�����ijɷ֣���Ӧǰ��E���м���������ͭ����16g����Ӧ����������������Ϊ13.6g����13.6g�ijɷ�Ϊ��___________����Ϊ�������������Ե�������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ����?Ӣ�Ŵ�����������У2010������¿��Ծ���һ�� ���ͣ�ʵ����
��ÿ��2�֣���12�֣�ijУ��ѧ�о���ѧϰС���ͬѧ��ѧϰ�˽�����֪ʶ��Ϊ�˽�Cu�ij������������ʣ��������ѧϰ��˼����� ���µ����⣬̽��������Э����С���ͬѧ��������о���
���µ����⣬̽��������Э����С���ͬѧ��������о���
��������⡿
��Cu�Ľ���������С��Al��Al(OH)3�������ԣ�Cu(OH)2Ҳ����������
��ͨ������£���2��Fe���ȶ���С�ڣ�3��Fe����1��Cu���ȶ���ҲС�ڣ�2��Cu�𣿢�CuO�ܱ�H2��CO�Ȼ�ԭ��Ҳ �ܱ�NH3��ԭ��
�ܱ�NH3��ԭ��
��ʵ�鷽����
��1�������������õ���ҩƷ�У�CuSO4��Һ���ߣߣߣߣߣߣߣߣߣ����Լ�����ͬʱ�������ʵ�顣
��2���������ڵ�ʵ�鲽����������£�ȡ98g Cu(OH)2���壬������80�桫100��ʱ���õ���ɫ�����ĩ���������ȵ�1000�����ϣ���ɫ��ĩȫ����ɺ�ɫ��ĩA����ȴ�����A������Ϊ72g����A�м���������ϡ���ᣬ�õ���ɫ��Һ��ͬʱ�۲쵽�������к�ɫ������ڡ���Щ���Ƶã�A�Ļ�ѧʽΪ�ߣߣߣߣߡ�
��3��Ϊ�������ۣ���Ƶ�ʵ��װ��Ϊ���гּ�β������װ��δ��������
ʵ���й۲쵽CuO��Ϊ��ɫ���ʣ���ˮCuSO4��ߣߣߣ�ɫ��ͬʱ����һ�ֶԴ�������Ⱦ�����塣
��ʵ����ۡ�
��1��Cu(OH)2�������ԣ�֤��Cu(OH)2�������Ե�ʵ������Ӧ�ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��2�����ݡ�ʵ�鷽����2�������ó��ģ�1��Cu�ͣ�2��Cu�ȶ��Դ�С�Ľ����ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
���������ۡ�
��1����ͬѧ��ΪNH3��CuO��Ӧ���ɵ� ��ɫ������Cu��Ҳ��ͬѧ��ΪNH3��CuO��Ӧ���ɵĺ�ɫ����
��ɫ������Cu��Ҳ��ͬѧ��ΪNH3��CuO��Ӧ���ɵĺ�ɫ���� ��Cu��A�Ļ����������һ����ʵ�����NH3��CuO��Ӧ�����ɵĺ�ɫ�������Ƿ���A�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��Cu��A�Ļ����������һ����ʵ�����NH3��CuO��Ӧ�����ɵĺ�ɫ�������Ƿ���A�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡΫ���������и�����ѧ���������Ͽ��Ի�ѧ�Ծ� ���ͣ�ʵ����
( 14��)Ϊ��̽��Ũ�ȶ����������Ե�Ӱ�죬ijѧϰС�����������̽�����
[̽��һ]��ȡ����������̼�ظ֣�12.0g����30.0mLŨ�����У����ȣ���ַ�Ӧ��õ���ҺX���ռ�������Y��
��1����ͬѧ��ΪX�г�Fe3+����ܺ���Fe2+����Ҫȷ�����е�Fe2+����ѡ�������Լ�����Ƽ�ʵ�鷽���������������̡�����ͽ��ۣ�
��
��ѡ���Լ���a.KSCN��Һ����ˮ b.���ۺ�KSCN��Һ
c.Ũ��ˮ d.����KMnO4��Һ
��2����ͬѧΪ�˲ⶨ���� Y��SO2�ĺ���������������ַ�����
Y��SO2�ĺ���������������ַ�����
����I.ȡ672mL����״��������Yͨ��������ˮ�У�Ȼ���������BaCl2��Һ�����ʵ�������ø������4.66g��
����II.��VmL c mol��L-1���Ը��������Һ�л���ͨ��Y����aL����״��������Һǡ����ȫ��ɫ��
����III��ȡVL����״��������Y����ͨ������������������Һ�У���ַ�Ӧ���ˡ�ϴ�ӡ���ɣ��Ƶù�������Ϊmg��
�����в������ķ����� �������� ��
��ѡ��������������ݼ�������Y��SO2���������
���ú�δ֪���Ĵ���ʽ��ʾ�� ��
[̽����]��������ʵ����SO2��������Ľ������ͬѧ��Ϊ����Y�л����ܺ�����H2��CO2���塣Ϊ�����������̽��ʵ��װ�ã�ͼ�мг�װ����ʡ�ԣ���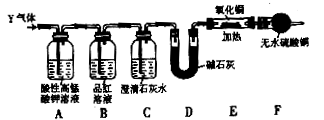
��3��װ��A���Լ��������� ��
��4������ȷ������Y�к���CO2��ʵ������ ��
��5����ͬѧ���ݡ�F�������ˮ����ͭ�Ƿ����ɫ��ȷ��Y�������Ƿ�������������Ϊ�Ƿ�ɿ��� ����ɿ������ɿ��������������ɣ�
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ֣���и�����һ������Ԥ�⻯ѧ�Ծ��������棩 ���ͣ�ʵ����
(12�֡�ij�о���ѧϰС����̽��SO2�ܷ���BaCl2��Һ��Ӧ����BaSO3�������������ϵ�֪������BaSO3��KSPΪ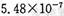 ��������������
��������������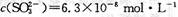 ��
��
(1) ��0.1 mol ? L��1��BaCl2��Һ���뱥���������У�_______ (��ܡ����ܡ�������BaSO3������ԭ����______________ (��д����Ҫ���ƶϹ��̣���
(2) Ũ����ķе�Ϊ338��C���ƾ��ƻ�����¶�Ϊ400?5000C����ͬѧ��װ��I����ʵ�飬����BaCl2��Һ�г��ְ�ɫ�������Ұ�ɫ�������������ᡣ
��д�������Թ��з�����Ӧ�Ļ�ѧ����ʽ��_____________________
�ڰ�ɫ�����Ļ�ѧʽ��_______���������ӷ���ʽ��ʾ���ɸð�ɫ�����Ŀ���ԭ��___________________________________
(3) ��ͬѧ��Ϊ��ͬѧ��װ�ò����ƣ�����˸Ľ�װ��II����ʵ�飨�г�װ�ú�A�м���װ�����ԣ��������Ѽ��飩��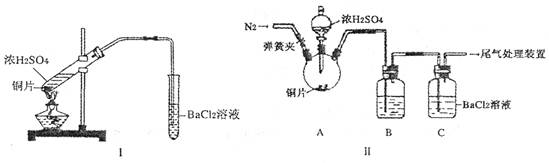
�ٴ��ɼУ�ͨ��N2����ʱ���رյ��ɼ�
�ڵμ�һ����Ũ���ᣬ����A��һ��ʱ���C��δ���������ɡ�
�����ٵ�Ŀ����_______��ϴ��ƿB�е��Լ���______________��
(4) ��ͬѧȡ��ʵ����C����Һ�������μ�һ����ɫ��Һ��Ҳ��������������İ�ɫ���������μӵ��Լ�������______________��
| A��NaOH��Һ | B��Na[Al(OH)4]��Һ | C��H2O2��Һ | D������ KMnO4��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ӱ�ʡ������ѧ�ڵڶ����¿���ѧ�Ծ� ���ͣ�ʵ����
(12��)ijѧϰС��������ͼ��ʾװ�ý��С�����ˮ����Ӧ��������ʵ��(��ȥ�˼г�����)��
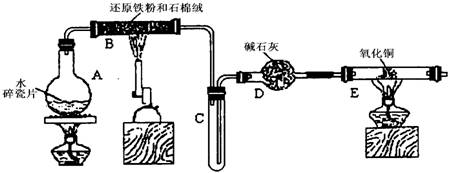
(1)Ϊ�˰�ȫ������E��ǰ������еIJ�����____________________________��
(2) B�з�����Ӧ�Ļ�ѧ����ʽ�� _______________��
(3)��֪�з�Ӧ��Cu2O+2H+ Cu+Cu2++H2O������������鷴Ӧ��E�еĺ�ɫ�����г�Cu���Ƿ�Cu2O�����õ��Լ���________(�����)
Cu+Cu2++H2O������������鷴Ӧ��E�еĺ�ɫ�����г�Cu���Ƿ�Cu2O�����õ��Լ���________(�����)
a��ϡ���� b��ϡ���� c������ d��Ũ����
(4)��E�����ٽӶ���ʢ��ʯ�ҵĸ����(���γ�ΪF��G)�����ø�װ�òⶨˮ����ɡ�����Ӧ��ʢ��ҩƷ��E������������e g��F������������f g��ˮ��Ԫ�ص����ʵ���֮�ȿɱ�ʾΪ�����û���n(H)��n(O)= ________��_______������Ӧ��E�г�Cu�������һ�ֻ�ԭ����Cu2O����ñ�ֵ��___________ (ѡ�ƫ��ƫС������Ӱ�족)��
(5)ijѧ��Ϊ�˼��鷴Ӧ�����ijɷ֣���Ӧǰ��E���м���������ͭ����16g����Ӧ����������������Ϊ13.6g����13.6g�ijɷ�Ϊ��___________����Ϊ�������������Ե�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08ï���ж�ģ��������12�֣�ijѧϰС��Ϊ̽��S��Cl2�ܷ�Ӧ���������ϻ����Ϣ�������Cl2��110�桫140����S��Ӧ���ɵ�S2Cl2��Ʒ���ɴ������������װ�ã��г�װ��δ����������ش�������⡣

(1)ʵ������У�ʹ�÷�Һ©���μ�Ũ����IJ����� ��2�֣���2���÷�Ӧ����ʽ��ʾHװ�õ����� ��2�֣���3��д��B�з�Ӧ�����ӷ���ʽ ��2�֣���4��C��D�е��Լ��ֱ��� �� ��2�֣���5��������Ʒ�к���SCl2��Ϊ����SCl2�����ɣ��ؼ��IJ����� ��2�֣���6��F���ܿ��ܻᷢ������������C��D֮�����һ��������װ�����ⷢ��Σ�ա���Ҫ��װ��ͼ������Լ����ƣ��г�װ�ò��軭���� ��2�֣�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com