
 ·ÖĪö¶ŌĖłÅäČÜŅŗÅضČÓ°Ļģ£®
·ÖĪö¶ŌĖłÅäČÜŅŗÅضČÓ°Ļģ£® ¼ÓÉīĄķ½ā£¬×¢ŅāĢ¼ĖįÄĘ³ĘĮæÓ¦·ÅŌŚŠ”ÉÕ±ÖŠ£¬ĶŠÅĢĢģĘ½µÄ¾«Č·¶ČĪŖ0.1£®
¼ÓÉīĄķ½ā£¬×¢ŅāĢ¼ĖįÄĘ³ĘĮæÓ¦·ÅŌŚŠ”ÉÕ±ÖŠ£¬ĶŠÅĢĢģĘ½µÄ¾«Č·¶ČĪŖ0.1£® 


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A | B | C | D | |
| Ćū³Ę | 250mlČŻĮæĘæ | ·ÖŅŗĀ©¶· | ĖįŹ½µĪ¶Ø¹Ü | ĪĀ¶Č¼Ę£ØĮæ³Ģ150”ę£© |
| Ķ¼ŠĪ | 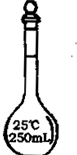 |
 |
 |
 |
| ÓĆĶ¾Óė²Ł×÷ | ÅäÖĘ1.0mol/LNaClČÜŅŗ¶ØČŻŹ±ŃöŹÓæĢ¶ČÅäµĆČÜŅŗÅضČ/1.0mol/L£® | ÓƱ½ŻĶČ”µāĖ®ÖŠµā·ÖŅŗŹ±£¬ÓĶ²ćŠč“ÓÉĻæŚ·Å³ö£® | æÉÓĆÓŚĮæČ”10.00mLµÄĖįŠŌKMnO4ČÜŅŗ£® | ÓĆÓŚŹµŃéŹŅÖĘŅŅĻ©Ź±£®²åČė·“Ó¦ŅŗæŲÖĘĖłŠčĪĀ¶Č£® |
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ×ßĻņĒå»Ŗ±±“óĶ¬²½µ¼¶Į”¤øßŅ»»Æѧ ĢāŠĶ£ŗ058
ÓĆÅضČĪŖ98£„£¬ĆܶČĪŖ1.84g/cm3µÄÅØH2SO4ÅäÖĘ0.2mol/LµÄĮņĖįČÜŅŗ250mL£®
(1)°“²Ł×÷µÄĻČŗóĖ³ŠņŠ“³öĖłŠčŅĒĘ÷µÄĆū³Ę________£®
(2)Ä³Ń§ÉśĶعż¼ĘĖćŗó½ųŠŠČēĻĀ²Ł×÷£ŗ
ÓĆĮæĶ²ĮæČ”ĆܶČĪŖ1.84g/cm3£¬ÅضČĪŖ98£„µÄÅØH2SO4 2.66mL£¬ĻņĮæĶ²ÖŠ¼ÓČėÉŁĮæÕōĮóĖ®£¬²¢ÓĆ²£Į§°ō½Į°č£¬Į¢¼“½«Ļ”ŹĶŗóµÄČÜŅŗÖ±½Óµ¹Čė250mLµÄČŻĮæĘæÖŠ£¬Č»ŗ󊔊ĵŲĶłČŻĮæĘæÖŠ¼ÓÕōĮóĖ®ÖĮæĢ¶ČĻߣ¬°ŃČŻĮæĘæøĒ½ōŅ”ŌČ£¬ŌŁ×°ČėŹŌ¼ĮĘæÖŠ£®
ŹŌÖø³öøĆÉś²Ł×÷ÖŠµÄ6“¦“ķĪó
¢Ł________________£»
¢Ś________________£»
¢Ū________________£»
¢Ü________________£»
¢Ż________________£»
¢Ž________________£®
(3)Īó²ī·ÖĪö£ŗĻĀĮŠĒéæöŹ¹ĖłÅäÖʵÄČÜŅŗÅØ¶Č±ČŹµ¼ŹÅضČĘ«“ó”¢Ę«Š”»¹ŹĒĪŽÓ°Ļģ£æ
A£®ÉÕ±”¢²£Į§°ōĪ“ÓĆÕōĮóĖ®Ļ“µÓ
[””””]
B£®ČŻĮæĘæŹ¹ÓĆĒ°ÓĆÕōĮóĖ®Ļ“ŗóĪ“øÉŌļ
[””””]
C£®Ī“ĄäČ“µ½ŹŅĪĀ¾Ķ½«ČÜŅŗ×ŖŅʵ½ČŻĮæĘæ
[””””]
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ0123 ĘŚÖŠĢā ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com