
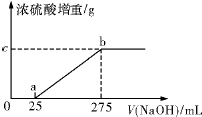
 ���������ƷA���ǣ�NH4��2SO4��NH4HSO4�Ļ���Ϊȷ��A�� ���ɷֵĺ�����ij�о���ѧϰС���ͬѧȡ��������ͬ��������ƷA����ˮ��Ȼ��ֱ���벻ͬ�����1mol/L��NaOH��Һ��ˮԡ���������� ȫ���ݳ������¶��£���β��ֽ⣩��������������������Ũ������ȫ�� �գ�Ũ�������ص����������NaOH��Һ������Ĺ�ϵ��ͼ��������ͼ�ش��������⣺
���������ƷA���ǣ�NH4��2SO4��NH4HSO4�Ļ���Ϊȷ��A�� ���ɷֵĺ�����ij�о���ѧϰС���ͬѧȡ��������ͬ��������ƷA����ˮ��Ȼ��ֱ���벻ͬ�����1mol/L��NaOH��Һ��ˮԡ���������� ȫ���ݳ������¶��£���β��ֽ⣩��������������������Ũ������ȫ�� �գ�Ũ�������ص����������NaOH��Һ������Ĺ�ϵ��ͼ��������ͼ�ش��������⣺
| ||
| ||
| ||
| 1mol/L��(0.275-0.025)L-0.025mol |
| 2 |


 ��ǰ����ϵ�д�
��ǰ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2013?������һģ���������������ƷA��B����ɷֶ��ǣ�NH4��2SO4��NH4HSO4�Ļ����ס��������о���ѧϰС���ͬѧ��Ҫȷ��A��B�и��ɷֵĺ�����
��2013?������һģ���������������ƷA��B����ɷֶ��ǣ�NH4��2SO4��NH4HSO4�Ļ����ס��������о���ѧϰС���ͬѧ��Ҫȷ��A��B�и��ɷֵĺ�����
| ||
| ||
| ʵ���� | �� | �� | �� | �� |
| ��������g�� | 9.88 | 19.76 | 29.64 | 49.40 |
| Ũ�������ӵ�������g�� | m | m | 1.36 | 0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���Ϻ��г����ظ�����ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�������
�������������ƷA��B����ɷֶ���(NH4)2SO4��NH4HSO4�Ļ����ס��������о���ѧϰС���ͬѧ��Ҫȷ��A��B�и��ɷֵĺ�����
����ͬѧȡ��������ͬ��������ƷA����ˮ��Ȼ��ֱ���벻ͬ�����1mol/L��NaOH��Һ��ˮԡ����������ȫ���ݳ��������¶��£���β��ֽ⣩��������������������Ũ������ȫ���ա�Ũ�������ص����������NaOH��Һ������Ĺ�ϵ��ͼ��������ͼ���ش��������⣺

��1��д��ab���漰�����ӷ���ʽ�� ��
��2��c���Ӧ����ֵ�� ����ƷA��(NH4)2SO4��NH4HSO4�����ʵ���֮��Ϊ ������ͬѧȡ�����ݲ�ͬ��������ƷB���ֱ���뵽200mL 1mol/L��NaOH��Һ�У�ͬ����ˮԡ���ȣ����ݳ�������������ŨH2SO4���ա��ⶨ������±���
|
ʵ���� |
�� |
�� |
�� |
�� |
|
��������g�� |
9.88 |
19.76 |
29.64 |
49.40 |
|
Ũ�������ӵ�������g�� |
m |
m |
1.36 |
0 |
�����ñ������ش��������⣺
��3���ٷ���ʵ�����ݿ�֪��ʵ����Ϊ ��ʵ���У�������������������е�笠���
����ȫת�������壻m��ֵΪ ��
�ڼ�����ƷB�е�Ԫ�ص���������������С����ʾ��������λС����
��1������ͬѧ���о�ʱ���֣�Ũ�������ص���������ƷB������֮������һ���ĺ�����ϵ��������Ʒ������Ϊx(g)��Ũ�������ص�����Ϊy(g)����x�ڲ�ͬ��Χʱy��x�ĺ�����ϵ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
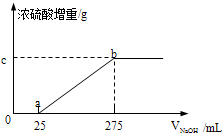 �������������ƷA��B����ɷֶ��ǣ�NH4��2SO4��NH4HSO4�Ļ����ס��������о���ѧϰС���ͬѧ��Ҫȷ��A��B�и��ɷֵĺ�����
�������������ƷA��B����ɷֶ��ǣ�NH4��2SO4��NH4HSO4�Ļ����ס��������о���ѧϰС���ͬѧ��Ҫȷ��A��B�и��ɷֵĺ�����| ʵ���� | �� | �� | �� | �� |
| ��������g�� | 9.88 | 19.76 | 29.64 | 49.40 |
| Ũ�������ӵ�������g�� | m | m | 1.36 | 0 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com