
”¾ĢāÄæ”æ“ÓĆŗŗĶŹÆÓĶÖŠæÉŅŌĢįĮ¶³ö»Æ¹¤ŌĮĻAŗĶB£¬AŹĒŅ»ÖÖ¹ūŹµ“ߏģ¼Į£¬ĖüµÄ²śĮæÓĆĄ“ŗāĮæŅ»øö¹ś¼ŅµÄŹÆÓĶ»Æ¹¤·¢Õ¹Ė®Ę½”£BŹĒŅ»ÖÖ±ČĖ®ĒįµÄÓĶדŅŗĢ¬Ģž£¬0.1 moløĆĢžŌŚ×ćĮæµÄŃõĘųÖŠĶźČ«Č¼ÉÕ£¬Éś³É0.6 mol CO2ŗĶ0.3 molH2O£»»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)AµÄµē×ÓŹ½________£¬BµÄ½į¹¹¼ņŹ½________”£
(2)µČÖŹĮæµÄA”¢BĶźČ«Č¼ÉÕŹ±ĻūŗÄO2µÄĪļÖŹµÄĮæA_______B(Ģī”°£¾”±”¢”°£¼”±»ņ”°£½”±)
(3)ÓėAĻąĮŚµÄĶ¬ĻµĪļCŹ¹äåµÄĖÄĀČ»ÆĢ¼ČÜŅŗĶŹÉ«µÄ»Æѧ·“Ó¦·½³ĢŹ½£ŗ_________________£¬·“Ó¦ĄąŠĶ£ŗ______________”£
”¾“š°ø”æ![]()
![]() £¾ CH2=CHCH3£«Br2”śCH2BrCHBrCH3 ¼Ó³É·“Ó¦
£¾ CH2=CHCH3£«Br2”śCH2BrCHBrCH3 ¼Ó³É·“Ó¦
”¾½āĪö”æ
AŹĒŅ»ÖÖ¹ūŹµ“ߏģ¼Į£¬ĖüµÄ²śĮæÓĆĄ“ŗāĮæŅ»øö¹ś¼ŅµÄŹÆÓĶ»Æ¹¤·¢Õ¹Ė®Ę½£¬ŌņAĪŖCH2=CH2£¬BŹĒŅ»ÖÖ±ČĖ®ĒįµÄÓĶדŅŗĢ¬Ģž£¬0.1moløĆĢžŌŚ×ćĮæµÄŃõĘųÖŠĶźČ«Č¼ÉÕ£¬Éś³É0.6mol CO2ŗĶ0.3molĖ®£¬øĆĢžÖŠN£ØC£©= 0.6mol/0.1mol=6”¢N£ØH£©= 0.3mol”Į2/0.1mol=6£¬¹ŹBµÄ·Ö×ÓŹ½ĪŖC6H6£¬BµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ78£¬Ōņ12n+2n-6=78£¬½āµĆn=6£¬ĖłŅŌBĪŖ±½”£
£Ø1£©AĪŖŅŅĻ©£¬½į¹¹¼ņŹ½ĪŖCH2=CH2£¬µē×ÓŹ½ĪŖ£ŗ![]() £»BĪŖ±½£¬½į¹¹¼ņŹ½ĪŖ
£»BĪŖ±½£¬½į¹¹¼ņŹ½ĪŖ![]() £¬¹Ź“š°øĪŖ£ŗ
£¬¹Ź“š°øĪŖ£ŗ![]() £»
£»![]() £»
£»
£Ø2£©ŅŅĻ©ÖŠHŌŖĖŲÖŹĮæ·ÖŹż±Č±½ÖŠHŌŖĖŲÖŹĮæ·ÖŹż“󣬹ŹĻąĶ¬ÖŹĮæµÄŅŅĻ©”¢±½Č¼ÉÕ£¬ŅŅĻ©ĻūŗĵÄŃõĘųøü¶ą£¬¼“µČÖŹĮæµÄA”¢BĶźČ«Č¼ÉÕŹ±ĻūŗÄO2µÄĪļÖŹµÄĮæA£¾B£¬¹Ź“š°øĪŖ£ŗ£¾£»
£Ø3£©ÓėAĻąĮŚµÄĶ¬ĻµĪļCĪŖCH2=CHCH3£¬CH2=CHCH3ÓėäåĖ®·¢Éś¼Ó³É·“Ó¦£¬Ź¹äåĖ®ĶŹÉ«£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗCH2=CHCH3+Br2”śCH2BrCHBrCH3£¬¹Ź“š°øĪŖ£ŗCH2=CHCH3+Br2”śCH2BrCHBrCH3£»¼Ó³É·“Ó¦”£


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÉčNAĪŖ°¢·ü¼ÓµĀĀŽ³£Źż£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(””””)
¢Ł±ź×¼×“æöĻĀ£¬11.2 LŅŌČĪŅā±ČĄż»ģŗĻµÄµŖĘųŗĶŃõĘųĖłŗ¬µÄŌ×ÓŹżĪŖNA
¢ŚĶ¬ĪĀĶ¬Ń¹ĻĀ£¬Ģå»żĻąĶ¬µÄĒāĘųŗĶė²ĘųĖłŗ¬µÄ·Ö×ÓŹżĻąµČ
¢Ū1 L 2 mol”¤L£1µÄAlCl3ČÜŅŗÖŠŗ¬ĀČĄė×ÓĪŖ6NA
¢Ü±ź×¼×“æöĻĀ£¬22.4 L H2OÖŠ·Ö×ÓŹżĪŖNA
¢Ż32 g O2ŗĶO3»ģŗĻĘųĢåÖŠŗ¬ÓŠŌ×ÓŹżĪŖ2NA
A. ¢Ł¢Ś¢Ū¢Ż B. ¢Ł¢Ś¢Ū¢Ü
C. ¢Ł¢Ū¢Ü D. ¢Ū¢Ü
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠĖµ·ØÖŠÕżČ·µÄŹĒ
A. Ļ”ÓŠĘųĢå±Č½ĻĪČ¶ØŹĒÓÉÓŚĘä·Ö×ÓÖŠĖłÓŠŌ×Ó×īĶā²ć¶¼Āś×ć8µē×ÓĪČ¶Ø½į¹¹
B. ŌŖĖŲÖÜĘŚ±ķÓŠÖ÷×唢ø±×唢Įć×åµČ¹²16ׯĮŠ
C. µŚĖÄÖÜĘŚµÄFe”¢Co”¢Ni¾łĪ»ÓŚµŚVIIIB×壬ĖłŅŌ»ÆѧŠŌÖŹĻąĖĘ
D. P3-¶ŌÓ¦ŌŖĖŲĪ»ÓŚµŚČżÖÜĘŚµŚVA×å
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŅŃÖŖA”¢B”¢C”¢D”¢E”¢FŹĒÖÜĘŚ±ķĒ°ĖÄÖÜĘŚµÄŌŖĖŲ£¬Ō×ÓŠņŹżŅĄ“ĪŌö“ó£¬A»łĢ¬Ō×ÓÖŠ£¬µē×ÓÕ¼¾ŻµÄ×īøßÄܲć·ūŗÅĪŖL£¬ĘäŗĖĶāÓŠĮ½¶Ō³É¶Ōµē×Ó£¬¼¤·¢Ģ¬×ī¶ąÓŠ4øö³Éµ„µē×Ó£¬CµÄ»łĢ¬Ō×ÓÖŠµē×ÓÕ¼¾ŻµÄ×īøßÄܲć·ūŗÅŅ²ĪŖL£¬pÄܼ¶ÉĻÓŠ4øöµē×Ó£»DŌ×ÓµÄ×īĶā²ćµē×ÓŹżÓėµē×Ó²ćŹżÖ®»żµČÓŚA”¢B”¢CČżÖÖŌŖĖŲµÄŌ×ÓŠņŹżÖ®ŗĶ£¬EŌ×ÓŗĖĶāµē×Ó×ÜŹż±ČĢśŌ×Ó¶ąĮ½øö£¬F»łĢ¬Ō×ÓµÄ×īĶāÄܲćÖ»ÓŠŅ»øöµē×Ó£¬ĘäĖūÄÜ²ć¾łŅŃ³äĀśµē×Ó”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)»łĢ¬FŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ______________________”£
(2)»łĢ¬EŌ×ӵļŪµē×ÓÅŲ¼Ź½ĪŖ______________________”£
(3)DC4£µÄæռ乹ŠĶŹĒ___________£¬ÓėDC4£»„ĪŖµČµē×ÓĢåµÄŅ»ÖÖ·Ö×ÓĪŖ___________(Ģī»ÆѧŹ½)£»HBC3ĖįŠŌ±ČHBC2Ē棬ĘäŌŅņŹĒ______________________”£
(4)A”¢B”¢CČżÖÖŌŖĖŲµŚŅ»µēĄėÄÜ×ī“óµÄŹĒ___________(ÓĆŌŖĖŲ·ūŗűķŹ¾)£¬ĘäŌŅņŹĒ_________________________________”£
(5)ĻņE(NO3)2ČÜŅŗÖŠÖšµĪ¼ÓČė°±Ė®£¬øÕæŖŹ¼Ź±Éś³ÉĀĢÉ«µÄE(OH)3³Įµķ£¬µ±°±Ė®¹żĮæŹ±£¬³Įµķ»įČܽā£¬Éś³É[E(NH3)6]2+µÄĄ¶É«ČÜŅŗ£¬Ōņ1mol[E(NH3)6]2+ŗ¬ÓŠµÄ¦Ņ¼üŹżÄæĪŖ___________”£
(6)D”¢NaŠĪ³ÉµÄŅ»ÖÖĄė×Ó»ÆŗĻĪļ£¬ŌŚČēĶ¼ĖłŹ¾¾§°ū½į¹¹Ķ¼ÖŠŗŚĒņ±ķŹ¾NaµÄĪ»ÖĆ£¬°×Ēņ±ķŹ¾DµÄĪ»ÖĆ£¬ŅŃÖŖøĆ¾§°ūµÄ±ß³¤ĪŖncm£¬°¢·ü¼ÓµĀĀŽ³£ŹżĪŖNA£¬¾§°ūµÄĆܶȦŃ=___________g/cm3(ÓĆŗ¬n”¢NAµÄ¼ĘĖćŹ½±ķŹ¾)”£

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÓŠ»śĪļWÓĆ×÷µ÷Ļć¼Į”¢øß·Ö×Ó²ÄĮĻŗĻ³ÉµÄÖŠ¼äĢåµČ£¬ÖʱøWµÄŅ»ÖÖŗĻ³ÉĀ·ĻßČēĻĀ”£

Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©FµÄ»ÆѧĆū³ĘŹĒ_______________£¬¢ŁµÄ·“Ó¦ĄąŠĶŹĒ_______________”£
£Ø2£©BÖŠŗ¬ÓŠµÄ¹ŁÄÜĶÅŹĒ_______________£ØŠ“Ćū³Ę£©£¬
£Ø3£©D¾ŪŗĻÉś³Éøß·Ö×Ó»ÆŗĻĪļµÄ»Æѧ·½³ĢŹ½ĪŖ________________”£
£Ø4£©·“Ó¦¢ŪµÄ»Æѧ·½³ĢŹ½ŹĒ___________________”£
£Ø5£©·¼Ļć»ÆŗĻĪļNŹĒAµÄĶ¬·ÖŅģ¹¹Ģ壬ĘäÖŠŗĖ“Ź²ÕńĒāĘ×ĪŖČż×é·åµÄ½į¹¹¼ņŹ½ĪŖ_________________”£
£Ø6£©·Ö×ÓŹ½C9H10O2µÄÓŠ»śĪļ£¬Ęä½į¹¹ÖŠŗ¬ÓŠ±½»·ĒŅæÉŅŌÓė±„ŗĶNaHCO3ČÜŅŗ·“Ó¦·Å³öĘųĢåµÄĶ¬·ÖŅģ¹¹ĢåÓŠ__________________ÖÖ(²»æ¼ĀĒĮ¢ĢåŅģ¹¹)”£
£Ø7£©²ĪÕÕÓŠ»śĪļWµÄÉĻŹöŗĻ³ÉĀ·Ļߣ¬Éč¼ĘŅŌMĪŖĘšŹ¼ŌĮĻÖʱøFµÄŗĻ³ÉĀ·Ļß(ĪŽ»śŹŌ¼ĮČĪŃ”)”£____________
![]()
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻÖŹ¹ÓĆĖį¼īÖŠŗĶµĪ¶Ø·Ø²ā¶ØŹŠŹŪ°×“×(Ö÷ŅŖ³É·ÖŹĒCH3COOH)µÄ×ÜĖįĮæ(g”¤100 mL£1)”£ŅŃÖŖCH3COOH + NaOH===CH3COONa + H2O ÖÕµćŹ±ĖłµĆČÜŅŗ³Ź¼īŠŌ”£
¢ń.ŹµŃé²½Öč£ŗ
(1)ÓĆŅĘŅŗ¹ÜĮæČ”10.00 mLŹ³ÓĆ°×“×£¬ŌŚÉÕ±ÖŠÓĆĖ®Ļ”ŹĶŗó×ŖŅʵ½100mL__________(ĢīŅĒĘ÷Ćū³Ę)ÖŠ¶ØČŻ£¬Ņ”ŌČ¼“µĆ“ż²ā°×“×ČÜŅŗ”£
(2)ÓĆ_____Č”“ż²ā°×“×ČÜŅŗ20.00 mLӌ׶ŠĪĘæÖŠ”£
(3)µĪ¼Ó2µĪ_____________×÷ÖøŹ¾¼Į”£
(4)¶ĮČ”Ź¢×°0.100 0 mol”¤L£1NaOH ČÜŅŗµÄ¼īŹ½µĪ¶Ø¹ÜµÄ³õŹ¼¶ĮŹż”£ Čē¹ūŅŗĆęĪ»ÖĆČēĶ¼ĖłŹ¾£¬Ōņ“ĖŹ±µÄ¶ĮŹżĪŖ________mL”£
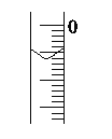
(5)µĪ¶Ø£¬µ±__________________Ź±£¬Ķ£Ö¹µĪ¶Ø£¬²¢¼ĒĀ¼NaOHČÜŅŗµÄÖÕ¶ĮŹż”£ÖŲø“µĪ¶Ø3“Ī”£
¢ņ.ŹµŃé¼ĒĀ¼
ŹµŃ鏿¾Ż£ØmL£© | 1 | 2 | 3 | 4 |
V(ѳʷ) | 20.00 | 20.00 | 20.00 | 20.00 |
V(NaOH)(ĻūŗÄ) | 15.95 | 15.00 | 15.05 | 14.95 |
¢ó.Źż¾Ż“¦ĄķÓėĢÖĀŪ£ŗ
(1)øł¾ŻÉĻŹöŹż¾Ż·ÖĪöµĆc(ŹŠŹŪ°×“×)£½________mol”¤L£1”£
(2)ŌŚ±¾ŹµŃéµÄµĪ¶Ø¹ż³ĢÖŠ£¬ĻĀĮŠ²Ł×÷»įŹ¹ŹµŃé½į¹ūĘ«Š”µÄŹĒ_______(ĢīŠ“ŠņŗÅ)”£
A£®¼īŹ½µĪ¶Ø¹ÜŌŚµĪ¶ØŹ±Ī“ÓƱź×¼NaOHČÜŅŗČóĻ“
B£®¼īŹ½µĪ¶Ø¹ÜµÄ¼ā×ģŌŚµĪ¶ØĒ°ÓŠĘųÅŻ£¬µĪ¶ØŗóĘųÅŻĻūŹ§
C£®×¶ŠĪĘæÖŠ¼ÓČė“ż²ā°×“×ČÜŅŗŗó£¬ŌŁ¼ÓÉŁĮæĖ®
D£®×¶ŠĪĘæŌŚµĪ¶ØŹ±¾ēĮŅŅ”¶Æ£¬ÓŠÉŁĮæŅŗĢ彦³ö
E. µĪ¶ØÖÕµć¶ĮŹżŹ±ø©ŹÓ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ£Ø1£©Š“³öĻĀĮŠø÷Ļī²Ł×÷µÄĆū³Ę£¬²¢Š“³öÓŠ±źŗŵÄŅĒĘ÷Ćū³Ę”£

A£ŗ²Ł×÷Ćū³Ę______________£»ŅĒĘ÷Ćū³Ę______________
B£ŗ²Ł×÷Ćū³Ę______________£»ŅĒĘ÷Ćū³Ę______________
C£ŗ²Ł×÷Ćū³Ę______________£»ŅĒĘ÷Ćū³Ę___________”¢______________”¢____________
D£ŗ²Ł×÷Ćū³Ę_____________£»ŅĒĘ÷Ćū³Ę________________
£Ø2£©ÅäÖĘ100ml”¢3.00mol/L NaClČÜŅŗ”£
¢Ł¼ĘĖćŠčŅŖNaCl¹ĢĢåµÄÖŹĮæ__________g”£
¢Śøł¾Ż¼ĘĖć½į¹ū£¬ÓĆĶŠÅĢĢģĘ½³Ę³ĘĮæNaCl¹ĢĢå__________g”£
¢Ū½«³ĘŗƵÄNaCl¹ĢĢå·ÅČėÉÕ±ÖŠ£¬ÓĆŹŹĮæÕōĮóĖ®Čܽā”£
¢Ü½«ÉÕ±ÖŠµÄČÜŅŗ×¢ČėČŻĮæĘ棬²¢ÓĆÉŁĮæÕōĮóĖ®_________________2~3“Ī£¬__________Ņ²¶¼×¢ČėČŻĮæĘ攣ĒįĒįŅ”¶ÆČŻĮæĘ棬Ź¹ČÜŅŗ»ģ¾ł”£
¢Ż½«ÕōĮóĖ®×¢ČėČŻĮæĘ棬ŅŗĆęĄėČŻĮæĘæ¾±æĢ¶ČĻßĻĀ________Ź±£¬øÄÓĆ______________µĪ¼ÓÕōĮóĖ®Ź¹ŅŗĆęÓėæĢ¶ČĻßĻąĒŠ£¬øĒŗĆĘæČū£¬ÉĻĻĀµßµ¹£¬Ņ”ŌČ”£
¢ŽÖü“ęČÜŅŗ”£
£Ø3£©ŹµŃéÖŠĖłÓĆ²£Į§ŅĒĘ÷³żĮæĶ²Ķā»¹ÓŠ________________________________________”£
£Ø4£©ĪŖŹ²Ć“ŅŖÓĆÕōĮóĖ®Ļ“µÓÉÕ±£¬²¢½«Ļ“µÓŅŗŅ²×¢ČėČŻĮæĘæ£æ“š£ŗ____________________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æøßĪĀĻĀ£¬Ä³·“Ó¦“ļĘ½ŗā£¬Ę½ŗā³£ŹżK=![]() ”£ŗćČŻŹ±£¬ĪĀ¶ČÉżøߣ¬H2ÅØ¶Č¼õŠ””£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
”£ŗćČŻŹ±£¬ĪĀ¶ČÉżøߣ¬H2ÅØ¶Č¼õŠ””£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
A”¢øĆ·“Ó¦µÄģŹ±äĪŖÕżÖµ
B”¢ŗćĪĀŗćČŻĻĀ£¬Ōö“óŃ¹Ē棬H2ÅضČŅ»¶Ø¼õŠ”
C”¢ÉżøßĪĀ¶Č£¬Äę·“Ó¦ĖŁĀŹ¼õŠ”
D”¢øĆ·“Ó¦»Æѧ·½³ĢŹ½ĪŖCO+H2O=CO2+H2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æČēĶ¼ŹĒ֊ѧ»Æѧ֊³£ÓĆÓŚ»ģŗĻĪļµÄ·ÖĄėŗĶĢį“æµÄ×°ÖĆ£¬Ēėøł¾Ż×°ÖĆ»Ų“šĪŹĢā£ŗ

£Ø1£©“ÓĀČ»Æ¼ŲČÜŅŗÖŠµĆµ½ĀČ»Æ¼Ų¹ĢĢå£¬Ń”Ōń×°ÖĆ___£ØĢī“ś±ķ×°ÖĆĶ¼µÄ×ÖÄø£¬ĻĀĶ¬£©£»³żČ„×ŌĄ“Ė®ÖŠµÄCl-µČŌÓÖŹ£¬Ń”Ōń×°ÖĆ___£¬¼ģŃé×ŌĄ“Ė®ÖŠCl-ŹĒ·ń³ż¾”µÄ·½·Ø£ŗ____”£
£Ø2£©“ÓµāĖ®ÖŠ·ÖĄė³öI2£¬Ń”Ōń×°ÖĆ___£¬øĆ·ÖĄė·½·ØµÄĆū³ĘĪŖ____”£
£Ø3£©×°ÖĆAÖŠ¢ŁµÄĆū³ĘŹĒ____£¬½ųĖ®µÄ·½ĻņŹĒ“Ó___æŚ½ųĖ®”£×°ÖĆBŌŚ·ÖŅŗŹ±ĪŖŹ¹ŅŗĢåĖ³ĄūĻĀµĪ£¬Ó¦½ųŠŠµÄ¾ßĢå²Ł×÷ŹĒ____”£
£Ø4£©ŗ£Ė®ÖŠŌĢ²Ų×Å·įø»µÄ׏Ō“£¬ŌŚŹµŃéŹŅ֊ȔɣĮæŗ£Ė®£¬½ųŠŠČēĶ¼Į÷³ĢµÄŹµŃé£ŗ
![]()
“ÖŃĪÖŠŗ¬Ca2+”¢Mg2+”¢Fe3+”¢SO42-µČŌÓÖŹ£¬ŠčŅŖĢį“æŗó²ÅÄÜ×ŪŗĻĄūÓĆ”£“ÖŃĪĢį“æµÄ²½ÖčÓŠ£ŗ
¢Ł¼ÓČė¹żĮæµÄNa2CO3ČÜŅŗ£» ¢Ś¼ÓČė¹żĮæµÄBaCl2ČÜŅŗ£»¢Ū¼ÓČė¹żĮæµÄNaOHČÜŅŗ£» ¢Üµ÷½ŚČÜŅŗµÄpHµČÓŚ7£» ¢ŻČܽā£» ¢Ž¹żĀĖ£» ¢ßÕō·¢”£
ÕżČ·µÄ²Ł×÷Ė³ŠņŹĒ____£ØĢīŠ“ŠņŗÅ£©”£
a£®¢Ż¢Ś¢Ū¢Ł¢Ž¢Ü¢ß b£®¢Ż¢Ł¢Ś¢Ū¢Ž¢Ü¢ß
c£®¢Ż¢Ś¢Ł¢Ū¢Ü¢Ž¢ß d£®¢Ż¢Ū¢Ś¢Ł¢Ž¢Ü¢ß
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com