
ЁОЬтФПЁПI.бЬЕРЦјжаЕФNO2ЪЧжївЊЕФДѓЦјЮлШОЮяжЎвЛЃЌЮЊСЫМрВтЦфКЌСПЃЌбЁгУШчЯТМьВтЗНЗЈЁЃЛиД№ЯТСаЮЪЬтЃК
НЋVLЦјбљЭЈШыЪЪСПЫсЛЏЕФH2O2ШмвКжаЃЌЪЙNO2ЭъШЋБЛбѕЛЏГЩNO3-ЃЌМгЫЎЯЁЪЭжС100.00mLЁЃСПШЁ20.00mLИУШмвКЃЌМгШыv1mLc1molЁЄLЃ1 FeSO4БъзМШмвК(Й§СП)ЃЌГфЗжЗДгІКѓЃЌгУc2molЁЄLЃ1 K2Cr2O7БъзМШмвКЕЮЖЈЪЃгрЕФFe2ЃЋЃЌжеЕуЪБЯћКФv2mLЁЃ
(1)NO2БЛH2O2бѕЛЏЮЊNO3-ЕФРызгЗНГЬЪНЮЊ____________________________________ЁЃ
(2)МгЫЎЯЁЪЭЕН100.00mlЫљгУЕФВЃСЇвЧЦїГ§СПЭВЁЂЩеБЁЂВЃСЇАєЁЂНКЭЗЕЮЙмЭтЃЌЛЙашвЊ__________________ЁЃ
(3)ЕЮЖЈЙ§ГЬжаЗЂЩњЯТСаЗДгІЃК
3Fe2ЃЋЃЋNO3-ЃЋ4HЃЋ=NOЁќЃЋ3Fe3ЃЋЃЋ2H2O
Cr2O72-ЃЋ6Fe2ЃЋЃЋ14HЃЋ=2Cr3ЃЋЃЋ6Fe3ЃЋЃЋ7H2O
дђЦјбљжаNO2ЕФКЌСПЮЊ___________mg/LЁЃ
(4)ЯТСаВйзїЛсЪЙЕЮЖЈНсЙћЦЋИпЕФЪЧ____
AЃЎЕЮЖЈЙмЮДгУБъзМвКШѓЯД BЃЎзЖаЮЦПЯДОЛКѓЛЙДцСєЩйСПЕФЫЎ
CЃЎЕЮЖЈЙмЕЮЖЈЧАЖСЪ§е§ШЗЃЌЕЮЖЈКѓИЉЪгЖСЪ§ DЃЎFeSO4БъзМШмвКВПЗжБфжЪ
IIЃЎГЃЮТЯТЃЌгУЗгЬЊзїжИЪОМСЃЌгУ0.10molЁЄLЃ1 NaOHШмвКЗжБ№ЕЮЖЈ20.00mLХЈЖШОљЮЊ0.10molЁЄLЃ1ЕФ CH3COOHШмвККЭHCNШмвКЫљЕУЕЮЖЈЧњЯпШчЭМЁЃ
ЃЈвбжЊЃКCH3COOHЁЂ HCNЕФЕчРыЦНКтГЃЪ§ЗжБ№ЮЊ1.75ЁС10-5ЁЂ6.4ЁС10-10ЃЉ
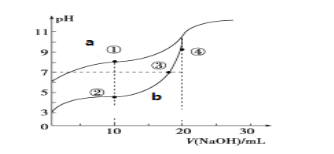
(1)ЭМ___ЃЈaЛђbЃЉЪЧNaOHШмвКЕЮЖЈHCNШмвКЕФpHБфЛЏЕФЧњЯпЃЌХаЖЯЕФРэгЩЪЧ_______________________ЁЃ
(2)ЕуЂлЫљЪОШмвКжаЫљКЌРызгХЈЖШЕФДгДѓЕНаЁЕФЫГађЃК_______________________ЁЃ
(3)ЕуЂйКЭЕуЂкЫљЪОШмвКжаЃКc(CH3COOЃ)Ѓc(CNЃ)___c(HCN)Ѓc(CH3COOH)ЃЈЬюЁА>ЁЂ<Лђ=ЁБЃЉ
(4)ЕуЂкЂлЂмЫљЪОЕФШмвКжаЫЎЕФЕчРыГЬЖШгЩДѓЕНаЁЕФЫГађЪЧЃК____________________ЁЃ
ЁОД№АИЁП2NO2ЃЋH2O2= 2NO3ЃЃЋ2HЃЋ 100mLШнСПЦП ![]() CD a HCNЕФЕчРыЦНКтГЃЪ§аЁЃЌЭЌХЈЖШЃЌЦфЕчРыГіЕФЧтРызгХЈЖШаЁЃЌpHжЕДѓ c(CH3COOЃ)= c(Na+) > c(OHЃ) = c(H+) = ЂмЂлЂк
CD a HCNЕФЕчРыЦНКтГЃЪ§аЁЃЌЭЌХЈЖШЃЌЦфЕчРыГіЕФЧтРызгХЈЖШаЁЃЌpHжЕДѓ c(CH3COOЃ)= c(Na+) > c(OHЃ) = c(H+) = ЂмЂлЂк
ЁОНтЮіЁП
I(1)NO2БЛH2O2бѕЛЏЮЊNO3ЃЕФРызгЗНГЬЪНЮЊ2NO2ЃЋH2O2= 2NO3ЃЃЋ2HЃЋЁЃ
(2)МгЫЎЯЁЪЭЕН100.00mlЫљгУЕФВЃСЇвЧЦїГ§СПЭВЁЂЩеБЁЂВЃСЇАєЁЂНКЭЗЕЮЙмЭтЃЌЛЙашвЊ100mLШнСПЦПЁЃ
(3)ЯШМЦЫужиИѕЫсИљРызгЯћКФЕУбЧЬњРызгЕФЮяжЪЕФСПЃЌдйМЦЫуЯѕЫсИљЯћКФЕУбЧЬњРызгЕФЮяжЪЕФСПЃЌдйМЦЯѕЫсИљЕФЮяжЪЕФСПМДЖўбѕЛЏЕЊЕФЮяжЪЕФСПЃЌдйМЦЫуvLЦјЬхжаЖўбѕЛЏЕЊЕФКЌСПЁЃ
(4)AбЁЯюЃЌЕЮЖЈЙмЮДгУБъзМвКШѓЯДЃЌЯћКФЕУБъвКЬхЛ§діДѓЃЌжиИѕЫсИљЬхЛ§ЖСЪ§ЦЋДѓЃЌжиИѕЫсИљЯћКФбЧЬњРызгЦЋДѓЃЌЯѕЫсИљЯћКФбЧЬњРызгЦЋаЁЃЌЕЮЖЈНсЙћЦЋЕЭЃЛBбЁЯюЃЌзЖаЮЦПЯДОЛКѓЛЙДцСєЩйСПЕФЫЎЃЌВтЖЈНсЙћВЛБфЃЛCбЁЯюЃЌЕЮЖЈЙмЕЮЖЈЧАЖСЪ§е§ШЗЃЌЕЮЖЈКѓИЉЪгЖСЪ§ЦЋаЁЃЌжиИѕЫсИљЯћКФбЧЬњРызгЦЋаЁЃЌЯѕЫсИљЯћКФбЧЬњРызгЦЋДѓЃЌЕЮЖЈНсЙћЦЋИпЃЛDбЁЯюЃЌFeSO4БъзМШмвКВПЗжБфжЪЃЌВтЖЈжиИѕЫсИљЬхЛ§ЦЋаЁЃЌжиИѕЫсИљЯћКФбЧЬњРызгЦЋаЁЃЌЯѕЫсИљЯћКФбЧЬњРызгЦЋДѓЃЌВтЖЈНсЙћЦЋИпЁЃ
IIЃЎ(1)ЭМИљОнЕчРыЦНКтГЃЪ§ПЩжЊЃЌЭЌХЈЖШHCNЕФpHЕчРыГЬЖШаЁЃЌpHДѓЃЌвђДЫЧњЯпaЪЧNaOHШмвКЕЮЖЈHCNШмвКЕФpHБфЛЏЕФЧњЯпЁЃ
(2)ЕуЂлЪЧДзЫсгыЧтбѕЛЏФЦЗДгІГЪжаадЃЌИљОнЕчКЩЪиКуКЭШмвКГЪжаадЕУГіРызгХЈЖШЕФДгДѓЕНаЁЕФЫГађЁЃ
(3)ЕуЂйШмжЪЮЊNaCNЁЂHCNЧвХЈЖШЯрЕШЃЌЕуЂкШмжЪЮЊCH3COOHЁЂCH3COONaЧвХЈЖШЯрЕШЃЌЕуЂйЁЂЕуЂкЕФШмжЪЕФХЈЖШЖМЯрЭЌЃЌИљОнЮяСЯЪиКуЕУБфаЮЕУГіНсТлЁЃ
(4)ДгЕуЂкЕНЕуЂмЙ§ГЬжаЃЌШмвКжавжжЦдйЗЂЩњЫсМюжаКЭЗДгІЃЌЫсЖдЫЎЕФЕчРыГЬЖШж№НЅМѕаЁЃЌЙЪЫЎЕФЕчРыГЬЖШж№НЅдіДѓЁЃ
I(1)NO2БЛH2O2бѕЛЏЮЊNO3ЃЕФРызгЗНГЬЪНЮЊ2NO2ЃЋH2O2= 2NO3ЃЃЋ2HЃЋЃЌЙЪД№АИЮЊЃК2NO2ЃЋH2O2= 2NO3ЃЃЋ2HЃЋЁЃ
(2) МгЫЎЯЁЪЭЕН100.00mlЫљгУЕФВЃСЇвЧЦїГ§СПЭВЁЂЩеБЁЂВЃСЇАєЁЂНКЭЗЕЮЙмЭтЃЌЛЙашвЊ100mLШнСПЦПЃЌЙЪД№АИЮЊЃК100mLШнСПЦПЁЃ
(3)Cr2O72ЃЃЋ 6Fe2ЃЋЃЋ14HЃЋ=2Cr3ЃЋЃЋ6Fe3ЃЋЃЋ7H2O
1mol 6mol
c2molЁЄLЃ1ЁСv2ЁС10-3 L xmol
![]()
x = 6c2v2ЁС10-3 mol
СђЫсбЧЬњЕФЮяжЪЕФСПn(FeSO4) = c1molЁЄLЃ1ЁСv1ЁС10-3 L= c1v1ЁС10-3 mol
ЯѕЫсИљбѕЛЏСђЫсбЧЬњЕФЮяжЪЕФСПЮЊn(FeSO4) = c1v1ЁС10-3 mol - 6c2v2ЁС10-3 mol =(c1v1- 6c2v2) ЁС10-3 mol
3Fe2ЃЋЃЋ NO3ЃЃЋ4HЃЋ=NOЁќЃЋ3Fe3ЃЋЃЋ2H2O
3mol 1mol
(c1v1- 6c2v2) ЁС10-3 mol ymol
![]()
![]()
вђДЫVLИУЦјЬхNO2ЕФЮяжЪЕФСП![]() ЃЌЦјбљжаNO2ЕФжЪСПЮЊ
ЃЌЦјбљжаNO2ЕФжЪСПЮЊ![]() =
=![]() ЃЌдђЦјбљжаNO2ЕФКЌСП
ЃЌдђЦјбљжаNO2ЕФКЌСП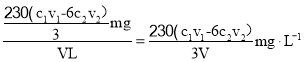 ЃЌЙЪД№АИЮЊЃК
ЃЌЙЪД№АИЮЊЃК![]() ЁЃ
ЁЃ
(4)AбЁЯюЃЌЕЮЖЈЙмЮДгУБъзМвКШѓЯДЃЌЯћКФЕУБъвКЬхЛ§діДѓЃЌжиИѕЫсИљЬхЛ§ЖСЪ§ЦЋДѓЃЌжиИѕЫсИљЯћКФбЧЬњРызгЦЋДѓЃЌЯѕЫсИљЯћКФбЧЬњРызгЦЋаЁЃЌЕЮЖЈНсЙћЦЋЕЭЃЛ
BбЁЯюЃЌзЖаЮЦПЯДОЛКѓЛЙДцСєЩйСПЕФЫЎЃЌВтЖЈНсЙћВЛБфЃЛ
CбЁЯюЃЌЕЮЖЈЙмЕЮЖЈЧАЖСЪ§е§ШЗЃЌЕЮЖЈКѓИЉЪгЖСЪ§ЦЋаЁЃЌжиИѕЫсИљЯћКФбЧЬњРызгЦЋаЁЃЌЯѕЫсИљЯћКФбЧЬњРызгЦЋДѓЃЌЕЮЖЈНсЙћЦЋИпЃЛ
DбЁЯюЃЌFeSO4БъзМШмвКВПЗжБфжЪЃЌВтЖЈжиИѕЫсИљЬхЛ§ЦЋаЁЃЌжиИѕЫсИљЯћКФбЧЬњРызгЦЋаЁЃЌЯѕЫсИљЯћКФбЧЬњРызгЦЋДѓЃЌВтЖЈНсЙћЦЋИпЃЛ
ЙЪД№АИЮЊCDЁЃ
IIЃЎ(1)ЭМИљОнЕчРыЦНКтГЃЪ§ПЩжЊЃЌЭЌХЈЖШЯТЃЌHCNЕФЕчРыГЬЖШаЁЃЌpHДѓЃЌвђДЫЧњЯпaЪЧNaOHШмвКЕЮЖЈHCNШмвКЕФpHБфЛЏЕФЧњЯпЃЌЙЪД№АИЮЊЃКaЃЛHCNЕФЕчРыЦНКтГЃЪ§аЁЃЌЭЌХЈЖШЃЌЦфЕчРыГіЕФЧтРызгХЈЖШаЁЃЌpHжЕДѓЁЃ
(2)ЕуЂлЪЧДзЫсгыЧтбѕЛЏФЦЗДгІГЪжаадЃЌИљОнЕчКЩЪиКуКЭШмвКГЪжаадЕУГіРызгХЈЖШЕФДгДѓЕНаЁЕФЫГађЃКc(CH3COOЃ)= c(Na+) > c(OHЃ) = c(H+)ЃЌЙЪД№АИЮЊЃКc(CH3COOЃ)= c(Na+) > c(OHЃ) = c(H+)ЁЃ
(3)ЕуЂйШмжЪЮЊNaCNЁЂHCNЧвХЈЖШЯрЕШЃЌЕуЂкШмжЪЮЊCH3COOHЁЂCH3COONaЧвХЈЖШЯрЕШЃЌЕуЂйЁЂЕуЂкЕФШмжЪЕФХЈЖШЖМЯрЭЌЃЌИљОнЮяСЯЪиКуЕУБфаЮЕУГіЃКc(CH3COOЃ)Ѓc(CNЃ) = c(HCN)Ѓc(CH3COOH)ЃЌЙЪД№АИЮЊЃК=ЁЃ
(4)ДгЕуЂкЕНЕуЂмЙ§ГЬжаЃЌШмвКжавжжЦдйЗЂЩњЫсМюжаКЭЗДгІЃЌЫсЖдЫЎЕФЕчРыГЬЖШж№НЅМѕаЁЃЌЙЪЫЎЕФЕчРыГЬЖШж№НЅдіДѓЃЌвђДЫШмвКжаЫЎЕФЕчРыГЬЖШгЩДѓЕНаЁЕФЫГађЪЧЃКЂмЂлЂкЃЌЙЪД№АИЮЊЃКЂмЂлЂкЁЃ


 дФЖСПьГЕЯЕСаД№АИ
дФЖСПьГЕЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПГфТњNO2КЭO2ЛьКЯЦјЬх30mLЕФЪдЙмЕЙСЂгкЫЎжаЃЌзюжеЪдЙмжаЪЃгр5mLЦјЬхЃЌдђдЪдЙмжаNO2КЭO2ЕФЬхЛ§БШПЩФмЪЧ
A.1ЉU1B.3ЉU1C.5ЉU1D.9ЉU1
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЗДгІЮяАДЫљИјЮяжЪЕФСПХфБШНјааЗДгІЃЌЦфжаЙЬЬхЭъШЋЗДгІЕФЪЧЃЈ ЃЉ
A.nЃЈCuЃЉЃКnЃЈHNO3ХЈЃЉ=1ЃК4B.nЃЈFeЃЉЃКnЃЈHNO3ХЈЃЉ=1ЃК2
C.nЃЈCЃЉЃКnЃЈH2SO4ХЈЃЉ=1ЃК2D.nЃЈMnO2ЃЉЃКnЃЈHClХЈЃЉ=1ЃК4
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПН№ЪєВФСЯдкШеГЃЩњЛюЁЂЩњВњжагазХЙуЗКЕФдЫгУЃЌЯТСаЙигкН№ЪєЕФЫЕЗЈВЛе§ШЗЕФЪЧ
A.ЙЄвЕЩЯН№ЪєMgЁЂAlЖМЪЧгУЕчНтШлШкЕФТШЛЏЮяжЦЕУЕФ
B.КЯН№ЕФаджЪгыЦфГЩЗжН№ЪєЕФаджЪВЛЭъШЋЯрЭЌ
C.Н№ЪєвБСЖЕФБОжЪЪЧН№ЪєбєРызгЕУЕНЕчзгБфГЩН№Ъєдзг
D.дНЛюЦУЕФН№ЪєдНФбвБСЖ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПАБДпЛЏбѕЛЏЗЈжЦЯѕЫсЪЧЙЄвЕЩњВњЯѕЫсЕФжївЊЭООЖЃЌФГЭЌбЇРћгУИУдРэдкЪЕбщЪвЬНОПЯѕЫсЕФжЦБИКЭаджЪЃЌЩшМЦСЫШчЭМЫљЪОзАжУЁЃ
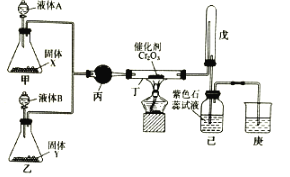
ЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉМзЁЂввСНзАжУЗжБ№жЦШЁАБЦјКЭбѕЦјЁЃЪЂзАвКЬхAЁЂBЕФвЧЦїУћГЦЮЊ__ЁЃШєЙЬЬхXЪЧNaOHЃЌдђвКЬхAЮЊ__ЃЈЬюУћГЦЃЉЃЛШєYЪЧЕЛЦЩЋЙЬЬхЁЂBЮЊДПОЛЮяЃЌдђвКЬхBЮЊ__ЃЈЬюЛЏбЇЪНЃЉЃЛШєЙЬЬхYЪЧMnO2ЃЌдђвКЬхBЮЊ__ЃЈЬюЛЏбЇЪНЃЉЁЃ
ЃЈ2ЃЉЖЁжаЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЮЊ__ЁЃ
ЃЈ3ЃЉзАжУЮьЕФзїгУЪЧ__ЁЃ
ЃЈ4ЃЉЮЊЗРжЙЙ§СПЕФАБЦјгАЯьЯѕЫсЕФжЦБИКЭаджЪЪЕбщЃЌПЩдкЖЁЁЂЮьжЎМфЬэМгвЛИіUаЭИЩдяЙмЃЌЦфжаЪЂзАЕФЪдМСПЩвдЪЧЯТСажаЕФ__ЁЃЃЈЬюБъКХЃЉ
A.МюЪЏЛв
B.ЩњЪЏЛв
C.ХЈСђЫс
D.ЮоЫЎТШЛЏИЦ
ЃЈ5ЃЉИФзАКѓЃЌЪЕбщжаЙлВьЕНЮьзАжУжаЕФЦјЬхБфЮЊКьзиЩЋЃЌМКЦПжаШмвКбеЩЋБфКьЁЃШєЭЈШыЕНМКЦПжаЕФКьзиЩЋЛьКЯЦјЬхЧЁКУгыЫЎЭъШЋЗДгІЧвЮоЦфЫћЦјЬхЩњГЩЃЌдђМКЦПжаЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЮЊ___ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПдквЛЖЈЬѕМўЯТФГЬхЛ§вЛЖЈЕФУмБеШнЦїжаЗЂЩњЕФЗДгІaA(g)+bB(g)![]() xC(g)ЗћКЯЯТЭММзЫљЪОЙиЯЕ(c%БэЪОЦНКтЛьКЯЦјЬхжаCЕФАйЗжКЌСПЃЌTБэЪОЮТЖШЃЌpБэЪОбЙЧП)ЁЃдђЭМввжазнжсyЪЧжИ
xC(g)ЗћКЯЯТЭММзЫљЪОЙиЯЕ(c%БэЪОЦНКтЛьКЯЦјЬхжаCЕФАйЗжКЌСПЃЌTБэЪОЮТЖШЃЌpБэЪОбЙЧП)ЁЃдђЭМввжазнжсyЪЧжИ
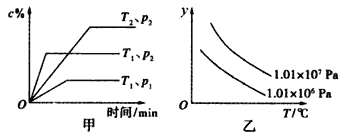
A. ЦНКтЛьКЯЦјЕФУмЖШ
B. ЦНКтЛьКЯЦјжаBЕФАйЗжКЌСП
C. ЦНКтЛьКЯЦјЕФзмЮяжЪЕФСП
D. ЦНКтЛьКЯЦјЕФЦНОљЯрЖдЗжзгжЪСП
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвЛЖЈЬѕМўЯТДцдкЗДгІЃКCO(g)ЃЋH2O(g)![]() CO2(g)ЃЋH2(g)ЃЌЦфе§ЗДгІЗХШШЁЃЯжгаШ§ИіЯрЭЌЕФ2 LКуШнОјШШ(гыЭтНчУЛгаШШСПНЛЛЛ)УмБеШнЦїЂёЁЂЂђЁЂЂѓЃЌдкЂёжаГфШы1 mol COКЭ1 mol H2OЃЌдкЂђжаГфШы1 mol CO2КЭ1 mol H2ЃЌдкЂѓжаГфШы2 mol COКЭ2 mol H2O,700 ЁцЬѕМўЯТПЊЪМЗДгІЁЃДяЕНЦНКтЪБЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧ(ЁЁЁЁ)
CO2(g)ЃЋH2(g)ЃЌЦфе§ЗДгІЗХШШЁЃЯжгаШ§ИіЯрЭЌЕФ2 LКуШнОјШШ(гыЭтНчУЛгаШШСПНЛЛЛ)УмБеШнЦїЂёЁЂЂђЁЂЂѓЃЌдкЂёжаГфШы1 mol COКЭ1 mol H2OЃЌдкЂђжаГфШы1 mol CO2КЭ1 mol H2ЃЌдкЂѓжаГфШы2 mol COКЭ2 mol H2O,700 ЁцЬѕМўЯТПЊЪМЗДгІЁЃДяЕНЦНКтЪБЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧ(ЁЁЁЁ)
A.ШнЦїЂёЁЂЂђжае§ЗДгІЫйТЪЯрЭЌB.ШнЦїЂёЁЂЂѓжаЗДгІЕФЦНКтГЃЪ§ЯрЭЌ
C.ШнЦїЂёжаCOЕФЮяжЪЕФСПБШШнЦїЂђжаЕФЩйD.ШнЦїЂёжаCOЕФзЊЛЏТЪгыШнЦїЂђжаCO2ЕФзЊЛЏТЪжЎКЭаЁгк1
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЪЕбщаЁзщРћгУNa2SO3КЭХЈСђЫсжЦБИSO2ВЂНјааЯрЙиЪЕбщЬНОПЁЃЪЕбщЗНАИШчЭМЁЃ
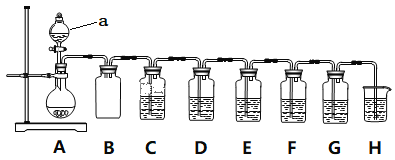
вбжЊЃКИїзАжУжаЕФЪдМСЃЌC(ЦЗКьШмвК)ЁЂD(ЫсадKMnO4ШмвК)ЁЂE(H2SШмвК)ЁЂF(ЕэЗлI2ЫЎШмвК)ЁЂG(H2O2КЭBaCl2ЛьКЯвК)
(1)вЧЦїзщзАЭъКѓЪзЯШвЊНјааЕФВйзїЪЧ____________________ЁЃ
(2)вЧЦїaЕФУћГЦЪЧ________________ЃЛзАжУBЕФзїгУ_____________________ЁЃ
(3)зАжУAжаЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪН____________________ЁЃ
(4)зАжУCКЭGжаЕФЯжЯѓЃКC___________________ЃЛG___________________ЃЛ
(5)ЩшМЦзАжУDКЭEЕФФПЕФЪЧбщжЄSO2ЕФ___________адКЭ___________адЁЃ
(6)зАжУHжаЕФЪдМСЪЧ______________ЃЛЦфзїгУЪЧ_____________________ЁЃ
(7)FжаЗДгІЕФРызгЗНГЬЪН____________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
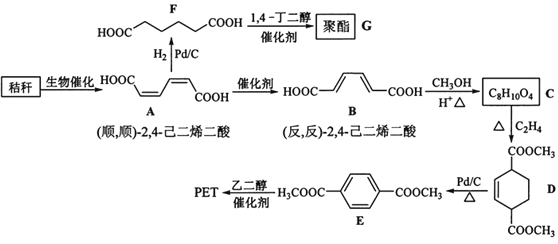
ЁОЬтФПЁПНеИб(КЌЖрЬЧЮяжЪ)ЕФзлКЯгІгУОпгаживЊЕФвтвхЁЃЯТУцЪЧвдНеИбЮЊдСЯКЯГЩОлѕЅРрИпЗжзгЛЏКЯЮяЕФТЗЯпЃК
ЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЯТСаЙигкЬЧРрЕФЫЕЗЈе§ШЗЕФЪЧ___ЁЃ(ЬюБъКХ)
a.ЬЧРрЖМгаЬ№ЮЖЃЌОпгаCnH2mOmЕФЭЈЪН
b.ТѓбПЬЧЫЎНтЩњГЩЛЅЮЊЭЌЗжвьЙЙЬхЕФЦЯЬбЬЧКЭЙћЬЧ
c.гУвјОЕЗДгІВЛФмХаЖЯЕэЗлЫЎНтЪЧЗёЭъШЋ
d.ЕэЗлКЭЯЫЮЌЫиЖМЪєгкЖрЬЧРрЬьШЛИпЗжзгЛЏКЯЮя
ЃЈ2ЃЉBЩњГЩCЕФЗДгІРраЭЮЊ___ЁЃ
ЃЈ3ЃЉDжаЙйФмЭХУћГЦЮЊ____ЃЌDЩњГЩEЕФЗДгІРраЭЮЊ___ЁЃ
ЃЈ4ЃЉFЕФЛЏбЇУћГЦЪЧ___ЃЌгЩFЩњГЩGЕФЛЏбЇЗНГЬЪНЮЊ___ЁЃ
ЃЈ5ЃЉОпгавЛжжЙйФмЭХЕФЖўШЁДњЗМЯуЛЏКЯЮяWЪЧEЕФЭЌЗжвьЙЙЬхЃЌ0.5molWгызуСПЬМЫсЧтФЦШмвКЗДгІЩњГЩ44gCO2ЃЌWЙВга___жж(ВЛКЌСЂЬхНсЙЙ)ЃЌЦфжаКЫДХЙВеёЧтЦзЮЊШ§зщЗхЕФНсЙЙМђЪНЮЊ____ЁЃ
ЃЈ6ЃЉВЮееЩЯЪіКЯГЩТЗЯпЃЌвд(ЗДЃЌЗД)-2ЃЌ4-МКЖўЯЉКЭC2H4ЮЊдСЯ(ЮоЛњЪдМСШЮбЁ)ЃЌЩшМЦжЦБИЖдБНЖўМзЫсЕФКЯГЩТЗЯп___ЁЃ
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com