

 �����[(NH4)2C2O4]����������ʡ�����ƣ�CaC2O4��������ˮ��Ca2+��
�����[(NH4)2C2O4]����������ʡ�����ƣ�CaC2O4��������ˮ��Ca2+�� ��
�� ����1�֣���2�֣�
����1�֣���2�֣�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����������������Ҵ� | B��AgNO3��KNO3��Na2CO3 |
| C��MnO2��CuO��FeO | D��(NH4)2SO4��K2SO4��NH4Cl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢� | B���ڢ� | C���ۢ� | D���ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������ױ�����������KMnO4��Һ�����Һ |
| B�����飨��ϩ����ͨ��ʢ����ˮ��ϴ��ƿ |
| C���Ҵ��������������÷�Һ©����Һ |
| D���������ӣ�����Ũ��ˮ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���屽(��)��NaOH��Һ����Һ | B����(����)��Ũ��ˮ������ |
| C���ƾ�(����ˮ)����ʯ�ң����� | D��������(�Ҵ�)��ˮ����Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

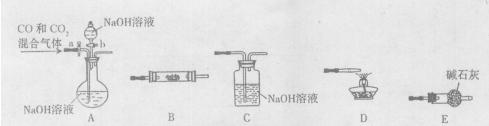
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��KMnO4��Һ | B��NaHCO3��Һ | C��NaOH��Һ | D��˫��ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ܢ� | B���ڢܢ� | C���ۢܢ� | D���٢ڢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com