
CaCO3�㷺��������Ȼ�磬��һ����Ҫ�Ļ���ԭ�ϡ�����ʯ��Ҫ�ɷ�ΪCaCO3�������������ĺ����ʵ�����ô���ʯ��ϡ���ᷴӦ�Ʊ�CO2���塣����װ�ÿ�����CO2������ᴿ���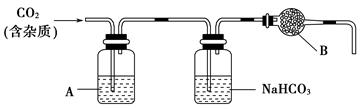
���������գ�
(1)��Ũ��������1��1(�����)��ϡ����(Լ6 mol��L��1)��Ӧѡ�õ�������________(����ĸ����ͬ)��
a���ձ������� b��������������c����Ͳ������ d������ƿ
(2)����װ���У�A��______��Һ��NaHCO3��Һ��������________________��
(3)����װ���У�B������________�������ʵ��õ�������ⶨCO2�ķ����������B����ʧЧ���ⶨ���________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
(4)һ���Է�����ʯ��(������)��CaCO3��ʳ���е��ܳ��������۷���������ָ��֮һ���ⶨ�ܳ�������Ҫʵ�鲽��������£����顢���ء������ܽ�����ˡ�������ɡ���ȴ�����ء����ء�Ϊ�˽�ʯ����̼����ܳ���Ӧѡ�õ��Լ���________��
a���Ȼ�����Һ���� b��ϡ���ᡡ��c��ϡ���ᡡ�� d��������
(5)���ܳ����ⶨʵ���У�Ϊ�˻��ʯ����̼��Ƶ�����ܳ�����Ӧ���ܳ�________�����ܳ�________��
(6)�����ⶨʵ���У�����________��˵����Ʒ�Ѿ����ء�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������������Ԫ��֮һ,����ֲ���纣���������к��зḻ�ġ��Ի���̬��ʽ���ڵĵ�Ԫ�ء���ʵ������,�Ӻ�������ȡ������̺�ʵ��װ������: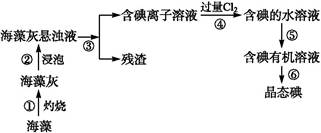
(1)ָ��������ȡ��Ĺ������й�ʵ�����������:�������������,���������������
(2)д������ܶ�Ӧ��Ӧ�����ӷ���ʽ:����������������������
(3)��ȡ��Ĺ�����,�ɹ�ѡ����л��Լ�������������(����)
A.�ƾ� B.���� C.���Ȼ�̼ D.��
(4)����ܳ��˼������Cl2,�������������ѡ����������(�����)��
A.Ũ���� B.H2O2��Һ C.KMnO4��Һ
������____________________________��
(5)Ϊ��ʹ������еĵ�����ת��Ϊ����л���Һ,����ɲ��������,ʵ���������ձ���������������ƿ���ƾ��ơ����ܡ�Բ����ƿ��ʯ�����Լ���Ҫ�ļг���������Ʒ,��ȱ�ٵIJ�������������������
(6)�Ӻ�����л��ܼ�����ȡ��ͻ����л��ܼ�,����Ҫ��������ָ����ͼʵ��װ���д��ڵĴ���֮��:���� 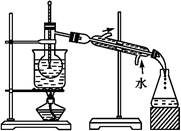
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijС��ͬѧ��һ��Ũ��NaHCO3��Һ���뵽CuSO4��Һ�з��������˳�������ͬѧ��Ϊ������CuCO3;��ͬѧ��Ϊ������CuCO3��Cu(OH)2�Ļ����,�������ʵ��ⶨ������CuCO3������������
(1)���ռ�ͬѧ�Ĺ۵�,������Ӧ�����ӷ���ʽΪ ��
(2)��ͬѧ������ͼ��ʾװ�ý��вⶨ:
�����о����������ǰ,�뽫��������Һ�з��벢�����������������Ϊ���ˡ�ϴ�ӡ����
��װ��E�м�ʯ�ҵ������� ��
��ʵ������������²�������:
a.�ر�K1��K3,��K2��K4,��ַ�Ӧ
b.��K1��K4,�ر�K2��K3,ͨ���������
c.��K1��K3,�ر�K2��K4,ͨ���������
��ȷ��˳���� (��ѡ�����,��ͬ)����δ���в��� ,��ʹ�������ƫ�͡�
����������Ʒ����Ϊm g,װ��D����������n g,�������CuCO3����������Ϊ ��
(3)��ͬѧ��Ϊ������ͨ������CO2����������� ���ⶨ������CuCO3������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������������C9H10O2������ˮ����ζ������������ˮ�㾫�����쾫�ͣ���������ʳƷ��ҵ�У�Ҳ�������л��ϳ��м��塢�ܼ��ȡ����Ʊ�����Ϊ��
��֪��
| | ��ɫ��״̬ | �е㣨�棩 | �� �ܶȣ�g��cm��3�� |
| *������ | ��ɫ��Ƭ״���� | 249 | 1.2659 |
| ���������� | ��ɫ����Һ�� | 212.6 | 1.05 |
| �Ҵ� | ��ɫ����Һ�� | 78.3 | 0.7893 |
| ������ | ��ɫ����Һ�� | 80.8 | 0.7318 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�о���ѧϰС��Ϊ�ϳ�1 ����,�������ϵ�֪һ���ϳ�·��:
����,�������ϵ�֪һ���ϳ�·��:
CH3CH CH2+CO+H2
CH2+CO+H2 CH3CH2CH2CHO
CH3CH2CH2CHO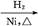 CH3CH2CH2CH2OH;
CH3CH2CH2CH2OH;
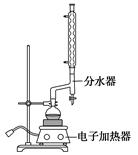
CO���Ʊ�ԭ��:HCOOH CO��+H2O,����Ƴ�ԭ�������Ʊ�װ��(��ͼ)��
CO��+H2O,����Ƴ�ԭ�������Ʊ�װ��(��ͼ)��
����д���пհ�:
(1)ʵ��������п����ϡ���ᡢϡ���ᡢŨ���ᡢ2 ����,����ѡ����ʵ��Լ��Ʊ���������ϩ,д����ѧ��Ӧ����ʽ:�� ,������������������������������
����,����ѡ����ʵ��Լ��Ʊ���������ϩ,д����ѧ��Ӧ����ʽ:�� ,������������������������������
(2)��������װ���Ʊ����﴿����CO,װ����a��b�����÷ֱ���������������,��������������c��d��ʢװ���Լ��ֱ��������������� ,���������� ����������װ���Ʊ�H2,���巢��װ���б���IJ�����������������������������;�����߿��ڻ����ռ�����H2��װ��ͼ��
(3)�Ʊ�ϩʱ,����������SO2��CO2��ˮ����,��С���������Լ���������������,�������ͨ���Լ���˳�������������������������� (�����)��
�ٱ���Na2SO3��Һ��������KMnO4��Һ����ʯ��ˮ������ˮCuSO4����Ʒ����Һ
(4)�ϳ�����ȩ�ķ�ӦΪ������ȵĿ��淴Ӧ,Ϊ����Ӧ���ʺ����ԭ������ת����,����ΪӦ�ò��õ����˷�Ӧ������������������
a.���¡���ѹ������
b.�ʵ����¶ȡ���ѹ������
c.���¡���ѹ������
d.�ʵ����¶ȡ���ѹ������
(5)����ȩ��������õ�����������ȩ��1 ������Ʒ��Ϊ����1
������Ʒ��Ϊ����1 ����,��С���������֪:��R��CHO+NaHSO3(����)
����,��С���������֪:��R��CHO+NaHSO3(����)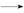 RCH(OH)SO3Na��;�ڷе�:����34��,1
RCH(OH)SO3Na��;�ڷе�:����34��,1 ���� 118��,����Ƴ������ᴿ·��:
���� 118��,����Ƴ������ᴿ·��:
��Ʒ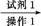 ��Һ
��Һ
 ���
���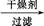 1
1 ����������
���������� ��Ʒ
��Ʒ
�Լ�1Ϊ����������,����1Ϊ��������,����2Ϊ��������,����3Ϊ��������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ż�����dzȺ�ɫ���壬�����Ҵ�������ˮ���㷺Ӧ����Ⱦ���������ҵ������������þ�ۺͼ״�Ϊԭ���Ʊ�ż������ʵ�鲽�����£�
����1���ڷ�Ӧ���м���һ���������������״���һС���⣬װ�������ܣ���������þ�ۣ�����������Ӧ��
����2���ȴ�þ�۷�Ӧ��ȫ���ټ���þ�ۣ���Ӧ�������У���þ����ȫ��Ӧ���Ȼ���30 min��
����3��������Һ����ȵ����ˮ�У������Ͻ��裬�ñ�����С���к���pHΪ4��5�������Ⱥ�ɫ���壬���ˣ���������ˮϴ�ӡ�
����4����95%���Ҵ��ؽᾧ��
��1������1�з�Ӧ����Ҫ���Ⱦ��ܾ��ҽ��У�ԭ����________________��þ�۷����μ��������һ����ȫ�������ԭ����___________________ _____________________________________________________��
��2������3�����������ñ�ˮϴ�ӵ�ԭ����________________����Ҫ���ռ״���ʵ������IJ��������������ܡ��ƾ��ơ�ţ�ǹܣ�Ӧ�ӹܣ�����ƿ�Ӧ��________________________________________________________________��
��3��ȡ0.1 gż����������5 mL���ҵı��У�����Һ�ֳ����ȷݣ��ֱ�װ�������Թ��У�����һ���Թ��ú�ֽ���÷�������������һ����������������䡣��ëϸ�ܸ�ȡ�������Թ��е���Һ���ھ�����ֽ��ĩ��1 cm�����ٽ���ֽ��ĩ�˽���װ��1��3�ı���������Һ�������У�ʵ���������������ͼ��ʾ��
��ʵ���з������������õķ�����________����
����ʵ������֪��________________�����ø÷��������ᴿ��ʽż������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
̼���ơ���������Ӻ���(aNa2CO3��bH2O2)����Ư�ס�ɱ�����á�ʵ�����á����������Ʊ������ʵ�ʵ�鲽�����£�
��1����ȡ����̼�����ܽ���һ����ˮ�������ƿ�У��ټ��������ȶ���(MgCl2��Na2SiO3)��������ȡ�
��2����������30%��H2O2��Һ�ڽ���״̬�µ�����ƿ�У���15 �����ҷ�Ӧ1 h��
��3������Ӧ��Ϻ��ټ���������ˮ�Ҵ������á��ᾧ�����ˡ�����ò�Ʒ��
(1)��1���У��ȶ�����ˮ��Ӧ�������ֳ�����������仯ѧ����ʽΪ___________________________________________________________��
(2)��2���У���Ӧ����Ϊ15 �����ҿɲ�ȡ�Ĵ�ʩ��_____________________
___________________________________________________��
(3)��3���У���ˮ�Ҵ���������____________________________________��
(4)H2O2�ĺ����ɺ�����Ʒ�����ӡ��ֳ�ȡm g(Լ0.5 g)��Ʒ��������й�������ˮ���Ƴ�250 mL��Һ��ȡ25.0 mL����ƿ�У�����ϡ�����ữ������c mol��L��1 KMnO4��Һ�ζ����յ㡣
������250 mL��Һ����IJ����������ձ�������������Ͳ________��________��
�ڵζ��յ�۲쵽��������______________________________________��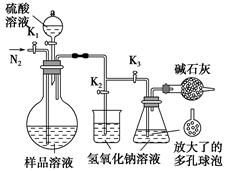
(5)��ģ�������ⶨ��Ʒ��̼���Ƶĺ�����װ������ͼ��ʾ(���Ⱥ̶�װ������ȥ)��ʵ�鲽�����£�
����1������ͼ��ʾ��װ���������װ�������ԡ�
����2��ȷ��ȡ(4)��������Һ50 mL����ƿ�С�
����3��ȷ��ȡ40.00 mLԼ0.2 mol��L��1 NaOH��Һ���ݣ��ֱ�ע���ձ�����ƿ�С�
����4������K1��K2���رջ���K3����ͨ�뵪��һ��ʱ��ر�K1��K2����K3������Һ©������ƿ�м���10 mL 3 mol��L��1������Һ��
����5����������ƿ�е�Һ����ڣ���������һ��ʱ�䡣
����6����K1�ٻ���ͨ�뵪��һ��ʱ�䡣
����7������ƿ�м������ָʾ������c1 mol��L��1 H2SO4����Һ�ζ����յ㣬����H2SO4����ҺV1 mL��
����8����ʵ�鲽��1��7�ظ����Ρ�
�ٲ���3�У�ȷ��ȡ40.00 mL NaOH��Һ����Ҫʹ�õ�������________��
�ڲ���1��7�У�ȷ�����ɵĶ�����̼������������Һ��ȫ���յ�ʵ�鲽����________(�����)��
��Ϊ�����Ʒ��̼���Ƶĺ��������貹���ʵ����______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ͼ1��ʾ��ʵ��������ȡ�����һ�ּ���װ�á�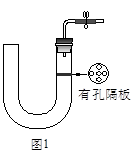
��1��������������������Եķ���_______________________��
��2��������ͼ1��ʾװ�ÿ�����ȡ���Ӧ��״����������Ӧ�Ƿ���Ҫ��������________________________���塣
ijͬѧ�����ͼ2��ʾװ�ã��ô�������16.9%ϡ���ᷴӦ��ȡNO���岢̽�����������ļ�̬����ش��й����⡣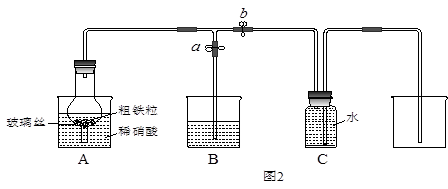
��3����֪16.9%ϡ������ܶ�Ϊ1.10g/cm3���������ʵ���Ũ��Ϊ____________��������������λС������������63%����������16.9%ϡ����500mL������IJ��������в��������ձ��� ��
��4������ֹˮ��a���ر�ֹˮ��bʱ��Aװ�õĸ�����й۲쵽��������_______________________��Bװ���ձ���Һ���������__________________________________����Aװ�������弸����ɫʱ����ֹˮ��b���ر�ֹˮ��a������Cװ���ռ�NO���塣
��5����֪���з�Ӧ���Է�����Fe2O3+3KNO3+4KOH 2K2FeO4+3KNO2+2H2O����Aװ���е�ϡ���ἴʹ����Ũ���ᣬҲ��������+6�۵����Ļ������ԭ����________��
2K2FeO4+3KNO2+2H2O����Aװ���е�ϡ���ἴʹ����Ũ���ᣬҲ��������+6�۵����Ļ������ԭ����________��
a��HNO3�������Ա�KNO3��
b����Ӧ���¶Ȳ���
c��HNO3�����ȶ��Ա�KNO3��
d��FeO42�����ܴ�����������Һ��
��6������������ҩƷ���Թܺͽ�ͷ�ιܣ�0.1mol/LKSCN��Һ��0.2mol/L����KMnO4��Һ��0.1mol/LKI��Һ����ˮ�ȡ��������һ����ʵ�飬̽��Aװ���ձ�����ȫ��Ӧ�������ܵļ�̬����д����ʵ�鱨�棺
| ʵ�鲽�� | ���� | ��������� |
| ��һ�� | ȡ����Һ��װ���Թܣ����� ���е��뼸��KSCN��Һ�� | |
| �ڶ��� | | ����Һ��ɫ��ȥ����˵������Fe2+�� �������Ա仯����˵������Fe2+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
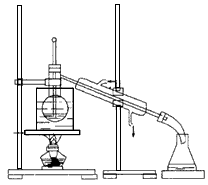
����ֲ���纣���������к��зḻ�ĵ�Ԫ�أ���Ԫ���Ե�������ʽ���ڡ�ʵ���ҴӺ�������ȡ����������£�
��1��ָ����ȡ��Ĺ������й�ʵ����������ƣ�
��Ϊ ����Ϊ �����̢����йط�Ӧ�����ӷ���ʽ�� ��
��2����ȡ��Ĺ����пɹ�ѡ����л��ܼ���( )
| A���ױ����ƾ� | B�����Ȼ�̼���� | C�����͡����� | D�����͡����� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com