
þ����Ͻ���һ����;�ܹ�Ľ������ϣ�Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ġ�ijѧУ������ȤС��Ӻ�ˮɹ�κ����±(��Ҫ��Na����Mg2����Cl����Br����)��ģ�ҵ��������ȡþ����Ҫ�������£�
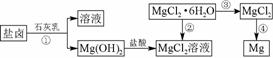
ͼ71
�ش��������⣺
(1)��ҵ�ϴ���±�л�ȡMg(OH)2��ʯ���������NaOH��Һ��ԭ����________________________________________________________________________��
(2)�ӹ��̢ٵõ���Mg(OH)2�����л���������Ca(OH)2����ȥ����Ca(OH)2�ķ������Ƚ��������뵽ʢ��________��Һ���ձ��У���ֽ����________��________(���������)�ɵô�����Mg(OH)2��
(3)ͼ72�Ǹ���ȤС����ƽ��й��̢۵�ʵ��װ��ͼ��
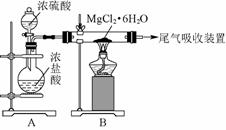
ͼ72
����װ��A��������_____________________________________________________��
(4)д�����̢��з�����Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________
________________________________________________________________________��
 Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й��ж���ȷ����(����)
A��0.1 mol��L��1Na2SO3��Һ����c(Na��)��2c(SO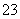 )��c(HSO
)��c(HSO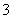 )��c(H2SO3)
)��c(H2SO3)
B��0.1 mol��L��1NH4Fe(SO4)2��Һ������Ũ�ȴ�С��ϵΪc(SO )>c(Fe3��)��c(NH
)>c(Fe3��)��c(NH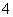 )>c(H��)>c(OH��)
)>c(H��)>c(OH��)
C��0.2 mol��L��1Na2CO3��Һ��0.1 mol��L��1NaHCO3��Һ�������Ϻ���c(Na��)��c(H��)��c(HCO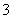 )��2c(CO
)��2c(CO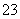 )��c(OH��)
)��c(OH��)
D��0.2 mol��L��1HA��Һ��0.1 mol��L��1NaOH��Һ�������ϵõ�����Һһ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ӵ��������ɷ��ӵĽṹ�����ģ�ͨ�������з��ӽṹ�Ĺ۲����Ʋ��������ʣ�
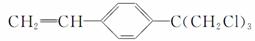
(1)�������ֿɷ���________��Ӧ��__________��Ӧ��
(2)��CH===CH2���ֿɷ���__________��Ӧ��__________��Ӧ��
(3)д�����л����γɵĸ߷��ӻ�����Ľṹ��ʽ��______________��
(4)���жϴ��л���ġ�C(CH2Cl)3����______(��ܡ����ܡ�)������ȥ��Ӧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������¶���ڸ���Ŀ����У��������仯����(����)
A��Na2O��������������B��Na2O2
C��NaHCO3 D��Na2CO3��10H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һ�������¶���ڿ�����һ��ʱ�䣬Ϊ����ù����Ƿֱ���Ϊ̼���ƣ��Ƚ�������Ʒ�ܽ���ˮ�õ���Һ������ȡ���д�ʩ�����п���ʵ��ʵ��Ŀ�ĵ���(����)
A����������Һ��pH
B��ȡ��Һ�����������е����̪�۲���Һ�Ƿ���
C��ȡ��Һ�����������м�������۲��Ƿ������ݲ���
D��ȡ��Һ�����������м���CuSO4��Һ���۲��Ƿ��г�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������ʵ����ͬһԭ�����͵���(�� ��)
A��Ũ�����Ũ���᳤�ڱ�¶�ڿ�����Ũ�Ƚ���
B����ˮ�ͻ���̿ʹ��īˮ��ɫ
C��Ư�ۺ�ˮ�������ڱ�¶�ڿ����б���
D��������̼�Ͷ���������ʹ����ʯ��ˮ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Һ�������ɺ����������ʹ������
A��AlCl3 B��KHCO3 C��Fe2(SO4)3 D��NH4HCO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Cu��CuO�Ļ����20.8g���뵽50mL 18.4mol��LŨH2SO4�У����ȳ�ַ�Ӧ������������ȫ�ܽ⣬��ȴ���Һϡ����1000 mL�����c��Cu2+��=0.3 mol��L���Լ��㣺
��1����Ӧ�����зų��������ڱ�״���µ��������������������Һ�е��ܽ⣩��
��2����Һϡ�ͺ�c��H+���Ƕ��٣�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com