
����ͨ��������ˮú���ķ����Ƶá�����CO��g��+ H2O��g�� CO2��g��+ H2��g��; ��H<0����850��ʱ��K=1��
��1���������¶ȵ�950��ʱ���ﵽƽ��ʱK �� 1������ڡ�����С�ڡ����ڡ���
��2��850��ʱ������һ�ݻ��ɱ���ܱ�������ͬʱ���� 1��0 mol CO��3��0molH2O��
1��0mol CO2 �� x mol H2����
�ٵ�x=5��0ʱ������ƽ���� �� ��������Ӧ���淴Ӧ��������С�
����Ҫʹ������Ӧ��ʼʱ������Ӧ������У���xӦ����������� �� ��
��3����850��ʱ������x��5��0 mol��x��6��0mol���������ʵ�Ͷ�ϲ��䣬��������Ӧ�ﵽƽ����H2����������ֱ�Ϊa����b������a �� b������ڡ�����С�ڡ����ڡ�����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| ||
| ||
| ||
| ||
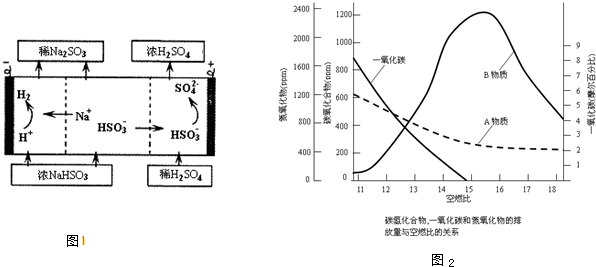
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011������ʡ�����и������������ۣ���ѧ���� ���ͣ������
��Դ�����ǵ�ǰ������������ٵ��������⣬ͬʱȫ�������ů����̬������������ͻ�����������ܡ�����ȼ�ϵ�ء���չ��̼�����ǻ�ѧ�����ߵ��о�����
I������ͨ��������ˮú���ķ����Ƶá�����CO(g)+H2O(g)  CO2(g)+H2(g)
CO2(g)+H2(g)
��H<0����850��ʱ��ƽ�ⳣ��K=1��
��1���������¶ȵ�750��ʱ���ﵽƽ��ʱK 1������ڡ�����С�ڡ����ڡ���
��2��850��ʱ������һ�ݻ��ɱ���ܱ�������ͬʱ����1.0molCO��3molH2O��1.0molCO2
��x molH2����
�ٵ�x=5.0ʱ��������Ӧ�� �������Ӧ�����淴Ӧ����������С�
����Ҫʹ������Ӧ��ʼʱ������Ӧ������У���xӦ����������� ��
����850��ʱ������x=5.0��x=6.0���������ʵ�Ͷ�ϲ��䣬��������Ӧ�ﵽƽ���
���H2����������ֱ�Ϊa%��b%����a b������ڡ�����С�ڡ����ڡ���
II����֪4.6gҺ̬�Ҵ���ȫȼ�����ɶ�����̼��Һ̬ˮ�ų�����136kJ��molҺ̬ˮת
��Ϊ����ˮ����44kJ��������
��3����д���Ҵ�ȼ��������̬ˮ���Ȼ�ѧ����ʽ
��
��4����0.1mol�Ҵ�������������ȼ�գ��õ�������ȫ��ͨ�뵽100mL3mol/LNaOH��Һ�У�����HCO-3�ĵ��룬��������Һ��c(CO2-3) c(HCO-3)������ڡ�����С�ڡ����ڡ�����ԭ���� ����������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ������ѧ�ڽμ�⻯ѧ�Ծ� ���ͣ������
�����ܡ�����δ�������������Դ��
��.ʵ���ã�1g����ȼ������Һ̬ˮʱ�ų�142.9kJ������������ȼ�յ��Ȼ�ѧ����ʽΪ_______��������ţ�
A.2H2��g��+O2��g��  2H2O��l��
��H= ��142.9kJ��mol��1
2H2O��l��
��H= ��142.9kJ��mol��1
B.H2��g��+1/2 O2��g��  H2O��l��
��H= ��285.8kJ��mol��1
H2O��l��
��H= ��285.8kJ��mol��1
C.2H2+O2 2H2O��l��
��H= ��571.6kJ��mol��1
2H2O��l��
��H= ��571.6kJ��mol��1
D.H2��g��+1/2
O2��g��  H2O��g�� ��H= ��285.8kJ��mol��1
H2O��g�� ��H= ��285.8kJ��mol��1
��.ij��ѧ�Ҹ��ݡ�ԭ�Ӿ��á���˼�룬����������Ʊ�H2�ķ�Ӧ����
��CaBr2+H2O CaO+2HBr ��2HBr+Hg
CaO+2HBr ��2HBr+Hg HgBr2+H2
HgBr2+H2
��HgBr2+_____ ______________
��2HgO
______________
��2HgO 2Hg+O2��
2Hg+O2��
������ݡ�ԭ�Ӿ��á���˼�������������۵Ļ�ѧ����ʽ��____________��
���ݡ���ɫ��ѧ����˼�������÷�����H2����Ҫȱ�㣺______________��
��.���ú��ܰ�ˮ�ֽ�����������Ŀǰ�����о��Ŀ��⡣��ͼ�����е�һ�����̣��������˹����ĵ⡣����ʾ����Ӧ�ڵIJ�����O2��SO2��H2O��
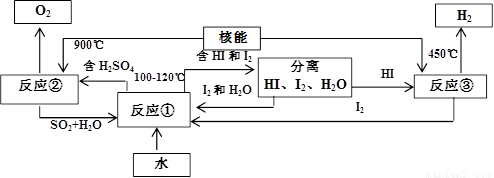
������з�Ӧ�Ļ�ѧ����ʽ��
��Ӧ��__________________________����Ӧ��__________________________��
�˷���ȡ����������ŵ���_______________________________________________��
��.����ͨ��������ˮú���ķ����Ƶá�����CO��g��+ H2O��g��  CO2��g��+ H2��g��; ��H<0��
CO2��g��+ H2��g��; ��H<0��
��850��ʱ��K=1��
��1���������¶ȵ�950��ʱ���ﵽƽ��ʱK______1������ڡ�����С�ڡ����ڡ���
��2��850��ʱ������һ�ݻ��ɱ���ܱ�������ͬʱ���� 1.0 mol CO��3.0molH2O��1.0mol CO2 �� x mol H2����
�ٵ�x=5.0ʱ������ƽ����___________��������Ӧ���淴Ӧ��������С�
����Ҫʹ������Ӧ��ʼʱ������Ӧ������У���xӦ�����������__________��
��3����850��ʱ������x��5.0 mol��x��6.0mol���������ʵ�Ͷ�ϲ��䣬��������Ӧ�ﵽƽ����H2����������ֱ�Ϊa����b������a _______ b������ڡ�����С�ڡ����ڡ�����
��.������ԭ����ͭ���õĺ�ɫ���������ͭ��������ͭ�Ļ�����֪Cu2O��������Һ�пɷ�������������ԭ��Ӧ������Cu2+�͵���ͭ��
��1������8������ͭ��������ԭ�õ���ɫ����6.8�ˣ����к�����ͭ��������ͭ�����ʵ���֮���� ��
��2������6.8�������������������ϡ�����ַ�Ӧ����ˣ��ɵõ����� g��
��3������6.8�������������һ������Ũ�����ַ�Ӧ��
�����ɱ�״����1.568�������壨������NO2���ܽ⣬Ҳ������NO2��N2O4��ת�������������ijɷ��� �������ʵ���֮���� ��
�ڰѵõ�����ҺС������Ũ�����������ľ�����ˣ��þ���23.68g����������ԭ��Һ�е�Cu2+��20%������ĸҺ�С������þ���Ļ�ѧʽ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com