


 Ņ»±¾ŗĆĢāæŚĖćĢāæØĻµĮŠ“š°ø
Ņ»±¾ŗĆĢāæŚĖćĢāæØĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ021
½«11gÓÉĮ½ÖÖ½šŹō×é³ÉµÄ»ģŗĻĪļĶ¶Čė×ćĮæĻ”ĮņĖįÖŠ£¬³ä·Ö·“Ó¦ŗóµĆµ½±ź×¼×“æöĻĀµÄĒāĘų11.2L£¬Ōņ½šŹō·ŪÄ©µÄ×é³É²»æÉÄÜŹĒ
[””””]
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ»Æѧ£ØøßŅ»ÉĻ£©µ„ŌŖĶ¬²½1£«1²āŹŌ¾ķ ĢāŠĶ£ŗ021
½«11gÓÉĮ½ÖÖ½šŹō×é³ÉµÄ»ģŗĻĪļĶ¶Čė×ćĮæĻ”ĮņĖįÖŠ£¬³ä·Ö·“Ó¦ŗóµĆµ½±ź×¼×“æöĻĀµÄĒāĘų11.2L£¬Ōņ½šŹō·ŪÄ©µÄ×é³É²»æÉÄÜŹĒ
[””””]
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģĖÄ“ØŹ”ÅŹÖ¦»ØŹŠøßČżµŚŅ»“ĪĶ³æ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
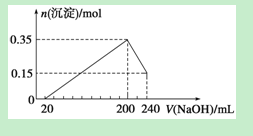
½«ÓÉMgŗĶAl×é³ÉµÄŅ»¶ØÖŹĮæµÄ»ģŗĻĪļĶ¶Čė500 mL Ļ”ĮņĖįÖŠ£¬¹ĢĢåČ«²æČܽā²¢²śÉśĘųĢ唣“ż·“Ó¦ĶźČ«ŗó£¬ĻņĖłµĆČÜŅŗÖŠ¼ÓČėNaOHČÜŅŗ£¬Éś³É³ĮµķµÄĪļÖŹµÄĮæÓė¼ÓČėNaOHČÜŅŗµÄĢå»ż¹ŲĻµČēÓŅĶ¼ĖłŹ¾”£ŌņĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
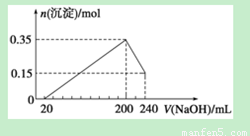
A£®ÉĻŹöÓÉMgŗĶAl×é³ÉµÄ»ģŗĻĪļµÄÖŹĮæĪŖ8g
B£®ĮņĖįµÄĪļÖŹµÄĮæÅضČĪŖ1 mol”¤L£1
C£®Éś³ÉµÄH2ŌŚ±ź×¼×“æöĻĀµÄĢå»żĪŖ11.2L
D£®NaOHČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ3.75 mol”¤L£1
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com