
 -﮻�ʯ����Ҫ�ɷ�ΪLi2O
-﮻�ʯ����Ҫ�ɷ�ΪLi2O Al2O3
Al2O3 4SiO2��Ϊԭ������
4SiO2��Ϊԭ������ ��Li2CO3�Ĺ����������£�
��Li2CO3�Ĺ����������£�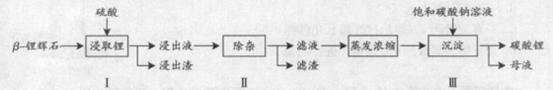
 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����


|

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 Au(NO3)3+3NO2��+ 3H2O����÷�Ӧ������еij̶ȼ�С�����Խ�������Ũ���ᣬ����ȴ����������ˮ���Լ�Ҫ����֮��
Au(NO3)3+3NO2��+ 3H2O����÷�Ӧ������еij̶ȼ�С�����Խ�������Ũ���ᣬ����ȴ����������ˮ���Լ�Ҫ����֮�� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����Ṥҵ |
| B���ϳɰ���ҵ |
| C�����Ṥҵ |
| D���ȼҵ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ѹ���� | B������ | C���ѽ� | D�����ѻ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ϳɰ���ҵѡ��ķ�Ӧ�����������£���500������ |
| B�������Ȼ�����Һʱ�����Ȼ������������У�Ȼ���ˮϡ�� |
| C��ʵ���ҳ����ű���ʳ��ˮ�ķ����ռ����� |
| D�����Ṥҵ�У�ʹ�ù����Ŀ�������߶�������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 2NH3(g) ���ڷ�Ӧ�����У�
2NH3(g) ���ڷ�Ӧ�����У� t1��t2��t3��t4ʱ�����ı䣬����Ӧ���ʷ����仯������ͼ�����ڿ��ܵ������ı������ж���ȷ����
t1��t2��t3��t4ʱ�����ı䣬����Ӧ���ʷ����仯������ͼ�����ڿ��ܵ������ı������ж���ȷ����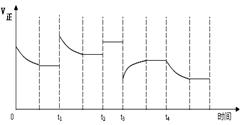
A��t1ʱ�����������¶� | B��t2ʱ����ʹ���˴��� |
C��t3ʱ���ܼ�С��ѹǿ | D��t4ʱ���ܽ������¶� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A��ͼIΪ�ϳɰ�������N2��H2ѭ��ʹ�� |
| B��ͼ��Ϊ�������MgCl2�õ�þ������HClѭ��ʹ�� |
| C��ͼ��Ϊ�������������ᣬ����NOѭ��ʹ�� |
| D��ͼI�����ں����Ƽ�ƴ�����CO2��ѭ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com