
����������ȼ�գ������������ֹ�̬������(P2O3��P2O5)��3.1 g�ĵ�����(P)��3.2 g������ȼ�գ�����Ӧ��ľ����ų�x kJ������
(1)ͨ������ȷ����Ӧ��������(�û�ѧʽ��ʾ)��_________��
����Ӧ������(g)�ֱ�Ϊ______________��
(2)��֪������ȼ����Ϊy kJ·mol��1����ʾ������ȼ�յ��Ȼ�ѧ����ʽΪ______________________________��
(3)����P2O3�������з�Ӧ���ɹ���P2O5���Ȼ�ѧ����ʽΪ________________________________________��
�𰸡�(1)P2O3��P2O5��2.75 g��3.55 g
(2)P(s)��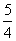 O2(g)===
O2(g)===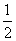 P2O5(s)��
P2O5(s)��
��H����y kJ·mol��1��
P(s)��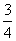 O2(g)===
O2(g)===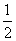 P2O3(s)��
P2O3(s)��
��H����(20x��y)kJ·mol��1��
(3)P2O3(s)��O2(g)===P2O5(s)��
��H��(40x��4y)kJ·mol��1
�������跴Ӧ������P2O3��P2O5�����ʵ����ֱ�Ϊm��n��
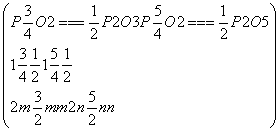
��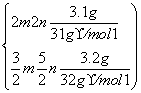
���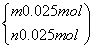
�ʿɷֱ����P2O5��P2O3��������
(2)������֪ȼ���ȼ�Ϊ1mol������P2O5ʱ�ķ�Ӧ�ȣ���P(s)��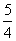 O2(g)===
O2(g)===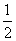 P2O5(s)����H����y kJ·mol��1��������0.025mol P2O5�ų�������Ϊ
P2O5(s)����H����y kJ·mol��1��������0.025mol P2O5�ų�������Ϊ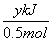 ��0.025mol��0.05y kJ��
��0.025mol��0.05y kJ��
1mol��������O2��Ӧ���ɹ���P2O3�ķ�Ӧ�ȣ�
��H��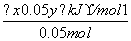 ��1mol����(20x��y)kJ·mol��1
��1mol����(20x��y)kJ·mol��1
(3)P(s)��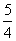 O2(g)===
O2(g)===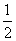 P2O5(s)��
P2O5(s)��
��H����y kJ·mol��1��
P(s)��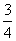 O2(g)===
O2(g)===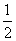 P2O3(s)��
P2O3(s)��
��H����(20x��y)kJ·mol��1��
���ݸ�˹���ɢ١�2���ڡ�2����P2O3(s)��O2(g)===P2O5(s)
��H��(40x��4y)kJ·mol��1��


 ����һ������ܼƻ�ϵ�д�
����һ������ܼƻ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ȼ����Ҫ�ɷ��Ǿ������ϩ
(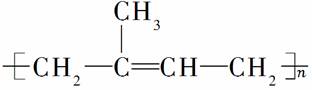 )������ϳ���Ҳ�����ƵĽṹ�������Լ����ϳ�ʱ��Ӵ����ᷢ����Ӧ����(����)
)������ϳ���Ҳ�����ƵĽṹ�������Լ����ϳ�ʱ��Ӵ����ᷢ����Ӧ����(����)
A�����Ը��������Һ
B����ˮ
C��Ũ����
D������������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Ϊ100 g��ͭ��Ϊ�缫�����������Һ�����һ��ʱ������缫�������28 g����ʱ��������Ϊ(����)
A��121.6 g B��88.2 g
C��89.6 g D��93.6 g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ӦA��B����C(��H<0)���������У���A��B����X(��H>0)����X����C(��H<0)��
����ʾ��ͼ�У�����ȷ��ʾ�ܷ�Ӧ�����������仯����(����)
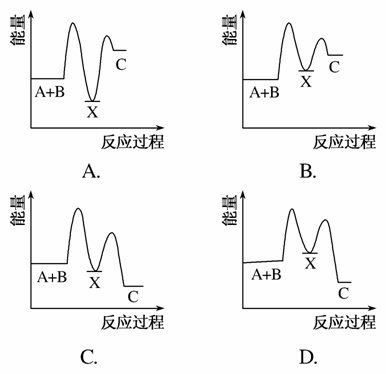
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������������ƾ���Ϊ����ȼ�ϵļ����Ѿ�����ȫ���ƹ㡣��֪C8H18��C2H5OHȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
2C8H18(l)��25O2(g)===16CO2(g)��18H2O(l)����H����10900 kJ·mol��1
C2H5OH(l)��3O2(g)===2CO2(g)��3H2O(l)�� ��H����1367 kJ·mol��1
�ٶ����͵ijɷ�ΪC8H18���������Ӿƾ�������������ȼ��ʱ�����ܴﵽ��Ŀ����(����)
A����ʡ��ʯȼ��
B�������к�������ŷ�
C��������ת����ʣ����ʳ
D�����ÿǧ��ȼ��ȼ�շų�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ཫ��δ����ʮ�������ɡ�̼��ȼ������ʱ������������̫��������ʱ����(�������ܡ��������ܵ�̫���ܵ�ת����̬)����ʱ���ǽ���Ӧ����̼���á��͡���̼���������˵������ȷ����(����)
A��ú��ʯ�ͺ���Ȼ��������̼��ȼ��
B����չ̫���ܾ��������ڼ�������ЧӦ
C��̫���ܵ�ؿɽ�̫����ֱ��ת��Ϊ����
D��Ŀǰ�о����˵����ʡ����硱������̫��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij����������������Ӧ��������ѭ���������ܽ��Ŀǰ��������Դ�����ij�����壺
3FeCl2��4H2O(g)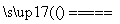 Fe3O4��6HCl��H2
Fe3O4��6HCl��H2
2Fe3O4��3Cl2��12HCl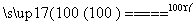 6FeCl3��6H2O��O2
6FeCl3��6H2O��O2
6FeCl3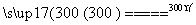 6FeCl2��3Cl2��
6FeCl2��3Cl2��
�ݴ���ش�
(1)����������������������________��
(2)�������������豸�������룬������������ֻ�����ӵ�ԭ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����пƬ�ʹ�ͭƬ��ͼʾ��ʽ����ͬŨ�ȵ�ϡ������һ��ʱ�䣬����������ȷ����(����)
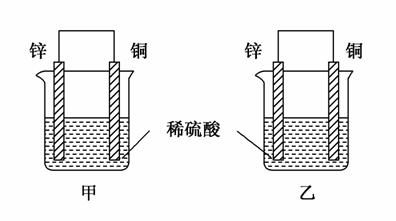
A�����ձ���ͭƬ����������ݲ���
B������ͭƬ������������ͭƬ�Ǹ���
C�����ձ�����Һ�����Ծ�����
D���������ݵ����ʼױ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ҹ���ѧ���ڡ�����(�������������黯����)���³����������о���ȡ������Ҫ�ɹ������о���Ŀ�ٻ�2013��ȡ�������Ȼ��ѧ����һ�Ƚ���
��1����̬Fe2+�ĺ�������Ų�ʽΪ_________________��
��2����������������Ԫ���е縺��ֵ�ɴ�С��˳����__________(����Ӧ��Ԫ�ط������)��
��3��Fe(SCN)3��Һ�м���NH4F���������·�Ӧ��Fe(SCN)3+6NH4F====(NH4)3FeF6+3NH4SCN��
��(NH4)3FeF6���ڵ����������������ۼ����_________(ѡ����ţ���ͬ)��
a. ��λ�� b. ��� c. ������ d. ���Ӽ�
����֪SCNһ�и�ԭ������������8�����ȶ��ṹ����Cԭ�ӵ��ӻ���ʽΪ_____________,��ԭ������ ����
���� �����ı�ֵΪ___________________��
�����ı�ֵΪ___________________��
��4��FeCl3����������ˮ���Ҵ����þƾ��Ƽ��ȼ�����������FeF3�����۵����1000oC���Խ������ֻ������۵����ϴ��ԭ��_______________________________��
��5�������ס�����Ϊͬ����Ԫ�أ����仯����Ľṹ�������Ƕ������ġ�
�ٸ����⻯��RH3(NH3��PH3��AsH3)��ij��������R�ĺ˵�����ı仯��������ͼ��ʾ����Y��ɱ�ʾ���⻯��(RH3)���ʿ�����________��
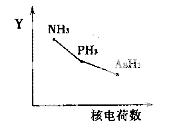
a���ȶ��� b���е� c ��R—H���� d�����Ӽ�������
��̼�����ѻ���������������ͺ��պ���������й㷺��Ӧ�ã���ṹ����̼ԭ��ȡ�������Ѿ���(�ṹ����ͼ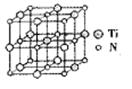 )����ĵ�ԭ�ӣ��ݴ˷���������̼�����ѻ�̨��Ļ�ѧʽΪ______��
)����ĵ�ԭ�ӣ��ݴ˷���������̼�����ѻ�̨��Ļ�ѧʽΪ______��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com