
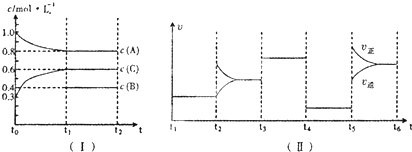
��һ��������ܱ������м���2 mol��A��0.6 mol��C��һ������B�������壮һ�������·�����Ӧ�������ʵ���Ũ����ʱ��仯��ͼ(��)��ʾ������t0��t1��c(B)δ������ͼ(��)Ϊt2ʱ�̺�ı䷴Ӧ��������ѧ��Ӧ������ʱ��仯��������ĸ��θı������������ͬ��ÿ����ֻ�ı�Ũ�ȡ�ѹǿ���¶ȡ������е�һ������������t3��t4��Ϊʹ�ô�����

��ش��������⣺
(1)��t1��15 min����t0��t1����C���ʵ�Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ________mol��L��1��min��1��
(2)t4��t5�θı������Ϊ________��B����ʼ���ʵ���Ũ��Ϊ________mol��L��1��
(3)t5��t6�α����������¶Ȳ��䣬��A�����ʵ������仯��0.01 mol�����˹����������������Ƚ�������Ϊa kJ��д�����¶��¸÷�Ӧ���Ȼ�ѧ����ʽ________��
 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 3 |
| 1 |
| 3 |
| 1 |
| 3 |
| 1 |
| 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| t1��t2 | t2��t3 | t3��t4�� | t4��t5 | t5��t6 |
| K1 | K2 | K3 | K4 | K5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ��������ܱ������м���![]() ��һ������B�������塣һ�������·�����Ӧ��������Ũ����ʱ��仯��ͼI��ʾ������
��һ������B�������塣һ�������·�����Ӧ��������Ũ����ʱ��仯��ͼI��ʾ������![]() ��
��![]() δ������ͼ��Ϊ
δ������ͼ��Ϊ![]() ʱ�̺�ı䷴Ӧ��������ѧ��Ӧ������ʱ��仯��������ĸ��θı������������ͬ��ÿ����ֻ�ı�Ũ�ȡ��¶ȡ�ѹǿ�������е�һ������������
ʱ�̺�ı䷴Ӧ��������ѧ��Ӧ������ʱ��仯��������ĸ��θı������������ͬ��ÿ����ֻ�ı�Ũ�ȡ��¶ȡ�ѹǿ�������е�һ������������![]() ��Ϊʹ�ô�����
��Ϊʹ�ô�����
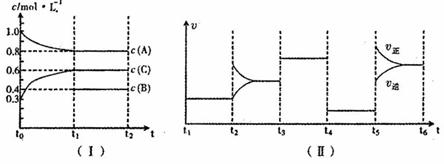
��ش��������⣺��1����![]() ����
����![]() ����C���ʵ�Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ_________��
����C���ʵ�Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ_________��
��2��![]() �θı������Ϊ___________��B����ʼ���ʵ���Ũ��Ϊ______________������ƽ��ʱ��Ӧ��ƽ�ⳣ�����±���ʾ��
�θı������Ϊ___________��B����ʼ���ʵ���Ũ��Ϊ______________������ƽ��ʱ��Ӧ��ƽ�ⳣ�����±���ʾ��
|
|
|
|
|
|
|
|
|
|
|
|
��![]() ��������λС������
��������λС������![]() ֮��Ĺ�ϵΪ____________���á�>������<����=�����ӣ���
֮��Ĺ�ϵΪ____________���á�>������<����=�����ӣ���
��3�� ![]() �α����������¶Ȳ��䣬��A�����ʵ������仯��
�α����������¶Ȳ��䣬��A�����ʵ������仯��![]() �����˹����������������Ƚ�������Ϊ
�����˹����������������Ƚ�������Ϊ![]() ��д�����¶��¸÷�Ӧ���Ȼ�ѧ����ʽ__________��
��д�����¶��¸÷�Ӧ���Ȼ�ѧ����ʽ__________��
��4������ͬ�����£�����ʼʱ�����м���![]() ��Ҫ�ﵽ
��Ҫ�ﵽ![]() ʱ��ͬ����ƽ�⣬
ʱ��ͬ����ƽ�⣬![]() Ҫ���������Ϊ________________��
Ҫ���������Ϊ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ɹŰ�ͷ��ʮ���и߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
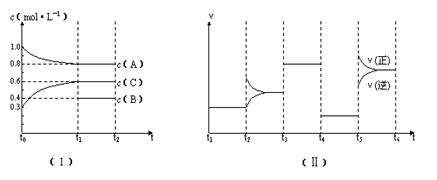
��10�֣�.��һ��������ܱ������м���2 mol A��0.6 mol C��һ������B�������塣һ�������·�����Ӧ�������ʵ���Ũ����ʱ��仯��ͼ������ʾ������t0--t1��c��B��δ������ͼ����Ϊt2ʱ�̺�ı䷴Ӧ��������ѧ��Ӧ������ʱ��仯 ��������ĸ��θı������������ͬ��ÿ����ֻ�ı�Ũ�ȡ�ѹǿ���¶ȡ������е�һ������������t3---t4��Ϊʹ�ô�����
��������ĸ��θı������������ͬ��ÿ����ֻ�ı�Ũ�ȡ�ѹǿ���¶ȡ������е�һ������������t3---t4��Ϊʹ�ô�����
��ش��������⣺
��1����t1="15" min����t0---t1����C���ʵ�Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ mol��  L��1��min��1��
L��1��min��1��
��2��t4--t5�θı������Ϊ ��B����ʼ���ʵ���Ũ��Ϊ mol�� L��1��
��3��t5----t6�α����������¶Ȳ��䣬��A�����ʵ������仯��0.01mol�����˹����е���ЧӦΪa kJ������д�����¶��¸÷�Ӧ���Ȼ�ѧ����ʽ

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ�差����У����-�߶���ѧ�����п��Ի�ѧ�� ���ͣ������
��֪��CO��g����H2O��g�� CO2��g����H2��g�� ��H= -42kJ��mol-1
CO2��g����H2��g�� ��H= -42kJ��mol-1
��1����һ���¶��£���һ��������ܱ�������ͨ��1molCO��2molH2O��g������Ӧ��ƽ��ʱ����÷ų�������Ϊ28kJ����CO��ת����Ϊ ��
��2�����¶��£�����ܱ�������ͨ��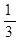 molCO2��
molCO2��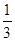 molH2����ʹ��Ӧ��ƽ��ʱCO��ת�����루1����ͬ�����������������
��
molH2����ʹ��Ӧ��ƽ��ʱCO��ת�����루1����ͬ�����������������
��
��3�����¶��£�����ܱ�������ͨ��2molCO��3molH2O��g������Ӧ��ƽ��ʱʱ����H2O��g����ת���� ��
��4�����¶��£�����ܱ�������ͨ��1.5molCO��3molH2O��g������Ӧ��ƽ��ʱ���ų�������Ϊ kJ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com