
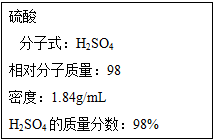
 ��ͼΪʵ����ijŨ�����Լ�ƿ�ı�ǩ���Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ı�ǩ���Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺���� ��1������c=$\frac{1000�Ѧ�}{M}$�������Ũ��������ʵ���Ũ�ȣ�
��2�����ƹ��������ʵ����ʵ������䣬����V=$\frac{n}{c}$�������Ҫ��Ũ����������
��3������һ�����ʵ���Ũ�ȵ���Һ����Ϊ�����㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ������ǩ���������Ʋ����ж���Ҫ��������ȱ�ٵ�������
��4�����ʵ���Ũ��c=$\frac{n}{V}$�����ݲ����������������ʵ���n����Һ���V��Ӱ�������������
��� �⣺��1����Ũ��������ʵ���Ũ��Ϊ��c=$\frac{1000�Ѧ�}{M}$=$\frac{1000��1.84��98%}{98}$mol/L=18.4 mol•L-1��
�ʴ�Ϊ��18.4 mol•L-1��
��2������250mL���ʵ���Ũ��Ϊ0.4mol•L-1��ϡ���ᣬ��Ҫ��������ʵ���Ϊ��0.4mol/L��0.25L=0.1mol�����ƹ�������������ʵ������䣬����Ҫ18.4mol/L��Ũ��������Ϊ��$\frac{0.1mol}{18.4mol/L}$��0.0054L=5.4mL��
�ʴ�Ϊ��5.4��
��3������250mL 0.4mol/L����Һ��һ�㲽��Ϊ�����㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ������ǩ����Ҫ������Ϊ����Ͳ���ձ�����������250mL����ƿ�ͽ�ͷ�ιܣ�����һ���õ����Ǣٲ��������ڽ�ͷ�ιܡ�����Ͳ�����ձ�����ȱ�ٵ�����Ϊ250mL����ƿ��
�ʴ�Ϊ���٢ڢۢߣ�250mL����ƿ��
��4���ٶ���ʱ���ӹ۲죬���¼��������ˮ���ƫС�����Ƶ���Һ���ƫС����Һ�����ʵ���Ũ��ƫ�ߣ��ʢ���ȷ��
�ڶ��ݺ���ҡ�ȡ����ú���Һ���½����������������ټ�����������ˮ���������Ƶ���Һ���ƫ��������ҺŨ��ƫ�ͣ��ʢڴ���
��Ũ�������ձ��м�ˮϡ�ͺ�δ��ȴ��������ƿ��ת�ƣ��ȵ���Һ���ƫ����ȴ����Һ���ƫС������������Һ���ƫС����Һ��Ũ��ƫ�ߣ��ʢ���ȷ��
������ƿʹ��ʱδ�����Ӱ�����ʵ����ʵ�������Һ�������Ӱ��������ҺŨ�ȣ��ʢܴ���
�ʴ�Ϊ���٢ۣ�
���� ���⿼��������һ�����ʵ���Ũ�ȵ���Һ�ķ�������Ŀ�ѶȲ������������ǿ�������߿���ע������ԣ����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ������˼ά�������Ͻ��Ĺ淶ʵ�����������������ѵ�������������ע����ȷ�������ķ����뼼�ɣ�


 ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaHCO3�TNa++H++CO32- | B�� | NaHSO4�TNa++H++SO42- | ||
| C�� | MgCl2�TMg2++2Cl- | D�� | Ca��OH��2�TCa2++2OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
| ��� | A | B | C |
�� �� װ �� |  |  |  |
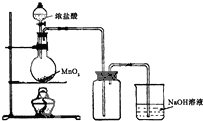
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ǵ���ʱ�����������ˮ��Һ���ۻ�״̬���ܷ���� | |
| B�� | ����ռ�����ڼ� | |
| C�� | ����ʱ�ܲ���H+�Ļ����һ������ | |
| D�� | �ǽ���������һ�������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 16g�����ͳ����Ļ�����к��е���ԭ����ΪNA | |
| B�� | ��״���£�22.4 Lˮ�к��е���ԭ����ΪNA | |
| C�� | 0.5mol•L-1��AlCl3��Һ������Al3+��ĿΪ0.5NA | |
| D�� | 0.1 mol ���������е�ԭ����Ϊ0.2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ɫʳƷ������ɫ��Ⱦ�����ೱ��������������ɫ�й� | |
| B�� | ��������Һ�����ڻ���������ҽ�þƾ�������Ƥ����������ԭ������ڿ�ʹ�����ʱ������� | |
| C�� | ������Գ��⡱��ϴ�Ӽ�����ȥ�͡����Ƿ����˻�ѧ�仯 | |
| D�� | ����ֲ���ͺͿ����͵ķ����Ǽ����������ռ���Һ�����ٷֲ��Ϊֲ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
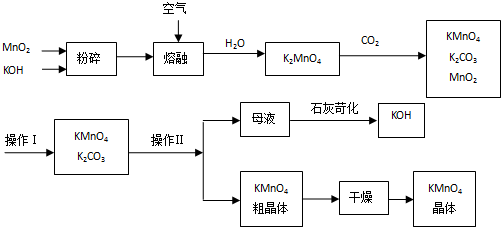
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
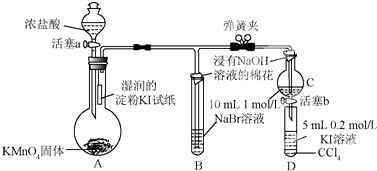
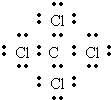 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com