
����Ŀ��I.ij��ѧ����С��������ͼһװ����ȡ�屽�������Һ©���� ���뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A��A�¶˻����رգ��С�
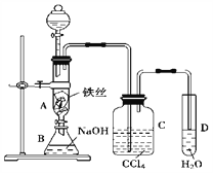
��1���۲쵽A�е�������_________________________________��
��2��ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������ д���йط�Ӧ�Ļ�ѧ����ʽ_____________________________��
��3��C��ʢ��CCl4��������________________________________��
��4����֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�м���AgNO3��Һ������������ɫ����������֤������һ����֤�ķ�����________________________________________��
II.ʵ��������ͼ��ʾ��װ����ȡ����������

��1���ڴ��Թ�������һ���������Ҵ��������Ũ����Ļ��Һ�������������£�__________��Ȼ������ʹ���Ͼ��ȡ�
��2��Ũ����������ǣ��� _______________���� ______________��
��3���ұ�װ����ͨ�����ĵ���Ҫ����Һ���϶����ܲ�����Һ�У�Ŀ���Ƿ�ֹ��Һ�ĵ�������ɵ�����ԭ����___________________��
��4������õ����������ķ�����________________��������Ҫ�IJ���������___________��
��5�����ӵ���C2H518OHд�������������ķ���ʽ________________________��
���𰸡� ��ӦҺ�У��к���ɫ�������A���� Br2+2NaOH=NaBr+NaBrO+H2O ��ȥ�廯�������е������� ʯ����Һ����Һ���ɫ ����Թ���ע�������Ҵ�����Ũ��������Ҵ��У��ӱ������������� ���� ��ˮ�� �ӷ������Ҵ�������������ˮ������ˮ����ѹǿ���������� ��Һ ��Һ©�����ձ� CH3COOH+CH3CH218OH====CH3CO18OCH2CH3+H2O
��������I.����Һ�������۴������·���ȡ����Ӧ�����屽���廯����C6H6+Br2![]() C6H5Br +HBr�����ڷ�Ӧ���ȣ�����Һ����ӷ������������屽���������廯��������ˮ�����H+��Br-�����������������ӻᷢ����Ӧ����AgBr������
C6H5Br +HBr�����ڷ�Ӧ���ȣ�����Һ����ӷ������������屽���������廯��������ˮ�����H+��Br-�����������������ӻᷢ����Ӧ����AgBr������
(1)���ڷ�Ӧ���ȣ�����Һ����ӷ�����������һ�ֺ���ɫ�����壬�ʴ�Ϊ����ӦҺ�У��к���ɫ�������A������
(2)A�е�����������Ʒ�Ӧ�����Խ��屽�е����ȥ����Br2+2NaOH=NaBr+NaBrO+H2O���ʴ�Ϊ��Br2+2NaOH=NaBr+NaBrO+H2O��
(3)������������ԭ�����弫���������Ȼ�̼�����廯�����ܣ�����C��ʢ��CCl4�������dz�ȥ�廯�������е����������ʴ�Ϊ����ȥ�廯�������е���������
(4)�������ȡ����Ӧ�������廯�⣬�廯��������ˮ�����H+��Br-��ֻҪ���麬�������ӻ������Ӽ��ɣ������ӵļ��飺ȡ��Һ�μ���������Һ��������ɵ���ɫ������֤���������ӣ������ӵļ��飺�����ʹ��ɫʯ����Һ��죬��֤�����������ӣ��ʴ�Ϊ��ʯ����Һ����Һ���ɫ��
II.(1)��ȡ�Ҵ��������Ũ����Ļ��Һʱ����ȷ��������Ϊ������Թ���ע�������Ҵ�����ŨH2SO4�����Ҵ��У��ӱ������������ᣬ�ʴ�Ϊ������Թ���ע�������Ҵ�����ŨH2SO4�����Ҵ��У��ӱ������������
(2)Ũ������������������ȡ��Ӧ�У��ܹ��ӿ췴Ӧ���ʣ����˴������ã��ܹ����շ�Ӧ���ɵ�ˮ��ͨ�������������IJ��ʣ�������ˮ�������ã��ʴ�Ϊ����������ˮ����
(3)�Ҵ�������ķе�ϵͣ���Ӧ���������ӷ������ڻӷ������Ҵ�������������ˮ������ˮ����ѹǿ��С���������ʴ�Ϊ���ӷ������Ҵ�������������ˮ������ˮ����ѹǿ��С��������
(4)�������������ڱ���̼������Һ�����Ի��Һ�ֲ㣬����ͨ����Һ�������������������������Һ����ʹ�õ������з�Һ©�����ձ��ȣ��ʴ�Ϊ����Һ����Һ©�����ձ���
(5)�������Ҵ��������������ķ�Ӧ�У��Ҵ���ȥ�ǻ��е���ԭ�ӡ�������ȥ�Ȼ��е��ǻ�������18O��Ӧ��������������У��÷�Ӧ�Ļ�ѧ����ʽΪCH3COOH+CH3CH218OH![]() CH3CO18OCH2CH3+H2O���ʴ�Ϊ��CH3COOH+CH3CH218OH
CH3CO18OCH2CH3+H2O���ʴ�Ϊ��CH3COOH+CH3CH218OH![]() CH3CO18OCH2CH3+H2O��
CH3CO18OCH2CH3+H2O��


 �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijУ��ѧ��ȤС��Ϊ�о�Cl2�����ʣ������ͼ��ʾװ�ý���ʵ��
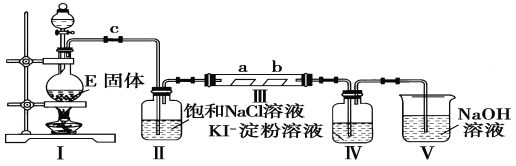
(1)ʵ�����Զ������̺�Ũ�����Ʊ������Ļ�ѧ����ʽΪ_______________��
(2)װ�â��������____________________________________��
(3)ʵ������У�װ�â��е�ʵ������Ϊ_________________________��������Ӧ�Ļ�ѧ����ʽΪ_______________________________��
(4)ʵ���������ͬѧ��װ�â���(a�Ǹ����Ʒ����ֽ��b�dz�ʪ��Ʒ����ֽ)�۲쵽b�ĺ�ɫ��ȥ�����Dz�δ�۲쵽��a�����Ա仯����һԤ������Ϊ�˴ﵽ��һʵ��Ŀ�ģ�����Ϊ��������ͼװ��_______��_____________֮��������ͼ�е�_____________װ��(�����)����װ�õ�������____________��
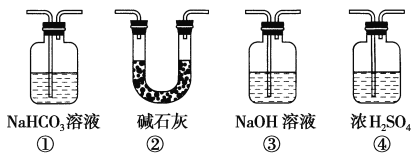
(5)װ��V��Ŀ���Ƿ�ֹβ����Ⱦ������д��װ��V�з�����Ӧ�Ļ�ѧ����ʽ��_________________��
(6)��8.7 g MnO2�뺬HCl 14.6 g��Ũ���Ṳ����Cl2����ͬѧ��Ϊ���Ƶ�Cl2 7.1 g����ͬѧ��Ϊ�Ƶ�Cl2������С��7.1 g������Ϊ__________(����������������)ͬѧ��ȷ��ԭ���� ___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ǰ���յ�ѧҵˮƽ����ʱ�ڣ����¶��轵������ѧ��ʹ�����ˡ�ů������ů�������õ������ġ��������ȡ�ԭ����ʹ�䷢��ԭ��ط�Ӧ����֪����ů������ԭ�������ۡ�ˮ������̿����ʯ��ʳ�Σ�������ԭ����г䵱
A. ���� B. ���� C. ���� D. ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��K2S2O8����ǿ������(S2O82-�к��й�����)����ʯ����ҵ������Ҫ��;����ҵ�Ͽ����õ�ⷨ�Ʊ���������ԭ����ͼ(�缫������ʯī)��ʾ������˵���д������
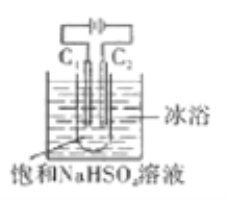
A. C1�缫�ϵĵ缫��ӦʽΪ��2SO42--2e-=S2O82-
B. ͨ���Na+����C2�缫
C. ��Ӧһ��ʱ�������Һ��pH��С
D. �������������缫�ϲ��뷴Ӧ�����ӵ����ʵ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ĸߵͿɺ���һ�����һ�������ˮƽ��չ�ĸߵ���SO2ת��ΪSO3���Ʊ������еĹؼ��Է�Ӧ��Ҳ��һ�����淴Ӧ��
��1��NO����ΪSO2��O2�䷴Ӧ�Ĵ�����������������
��2NO(g)+O2(g)![]() 2NO2(g)��H1=-113kJ/mol
2NO2(g)��H1=-113kJ/mol
��SO2(g)+NO2(g)![]() SO3(g)+NO(g) ��H2
SO3(g)+NO(g) ��H2
�ܷ�Ӧ��2SO2(g)+O2(g)![]() 2SO3(g)��H3=-196.6kJ/mol ��H2=__________
2SO3(g)��H3=-196.6kJ/mol ��H2=__________
��2��һ���¶��������ݻ�Ϊ2L�ĺ����ܱ������г���2molSO2��2mo1O2���������ѹǿ�ı仯���±���ʾ(SO3Ϊ����)��
��Ӧʱ��/min | 0 | 2 | 4 | 6 | 8 | 10 | 12 |
ѹǿ/MPa | 16.0a | 14.7a | 13.7a | 13.0a | 12.5a | 12.4a | 12.4a |
��0~10min������(SO2)=_________
�ڸ��¶��µ�ƽ�ⳣ��K=__(�������һλС��)��
�۷�Ӧ�ﵽƽ���������������ͬʱ�����Ϊ0.2mol���������������(��)����(��)�Ĺ�ϵ��___
��3��һ���¶�������ij�ܱ�������ͨ��һ�����Ķ�������������Ļ�����岢ʹ֮��Ӧ����Ӧ������SO2��O2��SO3���ʵ����仯����ͼ��ʾ��
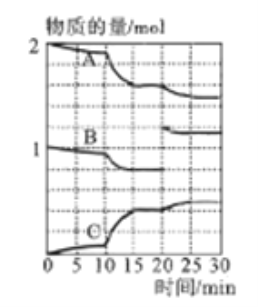
��A��B��C���������б�ʾSO2���ʵ����ı仯����__������15~20min��25~30min����ʱ����������ݻ�����������ijһʱ��SO3�ķֽ����ʽϴ��ʱ�����_______��
��10~15min�ڷ�Ӧ���ʷ��������Ա仯������Ե�ԭ����__________
(4)����������NaOH��Һ����SO2�ȿ�����SO2��ɵĴ�����Ⱦ��Ҳ�ɻ����Ҫ�Ļ�����Ʒ����ij����Һ��c(HSO3-):c(SO32-)=1:100����������Һ��pH=______(������K1(H2SO3)=1.5��10-2��K2(H2SO3)=1��10-7
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и����У����ܲ���������ﵽ2n2����(����)
A. Ne��Ar B. F����Mg2��
C. Al��O2�� D. Cl����Ar
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����б仯�У������ڻ�ѧ�仯���ǣ� ��
A.ú�ĸ���B.�����ѻ�C.ú��Һ��D.ʯ�ͷ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��S2Cl2�����ĵ�������ճ����������ڵ�����ͨ������������Ӧ�Ƶã���һ���Ȼ��ɵ�SCl2��S2Cl2��SCl2��ijЩ�������£�
ˮ���� | �ܶ�(g/cm3) | ��ɫ | �۵� | �е� | |
S2Cl2 | �����з���������ˮ��ˮ�� | 1.687 | ���ɫ | -76�� | 138�� |
SCl2 | ����ˮ�Ҿ��ҷ�Ӧ | 1.621 | ӣ�Һ� | -122�� | 59�� |
����ͼ��ʾװ���Ʊ�S2Cl2���ش��������⣺
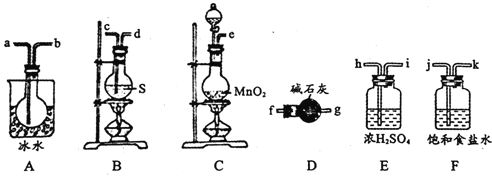
(1)��֪S2Cl2�����и�ԭ������������8�����ȶ��ṹ����S2Cl2�ĵ���ʽΪ________��
(2)��ȡCl2Ӧѡ�õ��b����_______(����ĸ���)����Ӧ�����ӷ���ʽΪ________��
(3)���õ��ϴ�����S2Cl2����������װ�õ�����˳��Ϊ____(������������Сд��ĸ��ʾ)��
(4)����D��������_______��D�м�ʯ�ҵ�������________��
(5)Ϊ�˻�ø�������S2Cl2����Ҫ�Բ�Ʒ���еIJ�����____________
(6)����S2Cl2����ˮ��ͬʱ�������������壬д����Ӧ�Ļ�ѧ����ʽ_________���÷�Ӧ�б������ͱ���ԭ��Ԫ�ص�����֮��Ϊ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪H2(g)��I2(g)![]() 2HI(g)��H<0������ͬ�ݻ��Ķ����ܱ��������ң����м���H2��I2��0.1mol�����м���HI0.2mol������ͬ�¶��·ֱ�ﵽƽ�⡣����ʹ����HIƽ��ʱ�İٷֺ�����������HIƽ��ʱ�İٷֺ�������Ӧ��ȡ�Ĵ�ʩ��
2HI(g)��H<0������ͬ�ݻ��Ķ����ܱ��������ң����м���H2��I2��0.1mol�����м���HI0.2mol������ͬ�¶��·ֱ�ﵽƽ�⡣����ʹ����HIƽ��ʱ�İٷֺ�����������HIƽ��ʱ�İٷֺ�������Ӧ��ȡ�Ĵ�ʩ��
A. �ס��������ͬ�¶� B. ���м���0.1molHe�����в���
C. �����¶ȣ�������ѹǿ D. ������0.1molH2��������0.1molI2
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com