
| A���٢ۢ� | B���ڢܢ� | C���٢ۢ� | D���ۢݢ� |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��װ����Һ����ƿ��������ˮ |
| B���ζ��ܼ��첿���ڵζ�ǰ������ |
| C��ʢ����ĵζ���δ�ñ�����ϴ |
| D���ζ�ǰ���Ӷ������ζ����Ӷ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ǰ�ȵ���������ƽ����� |
| B������ʱ���̷ű���������̷����� |
| C����ʪ�Ļ���и�ʴ�Ե�ҩƷ��������ڲ����������������������ҩƷ��ֱ�ӷ�����ƽ�����ϳ��� |
| D����������ƽ����ȷ������0.01g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A��250 mL����ƿ | B��������ƽ | C����ͷ�ι� | D���ձ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ζ���ϴ��������ˮ��ϴ������ע���Һ���еζ� |
| B����������պˮ����ת������Ƥ���в��� |
| C�����������е�����ͭ���壬ʧˮ�����ڸ���������ȴ���ٳ��� |
| D��������պȡ��Һ�ε����ڱ������ϵ�pH��ֽ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A���ڢۢܢݢ� | B���ڢܢݢޢ� | C���٢ܢݢޢ� | D���ڢۢܢݢޢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
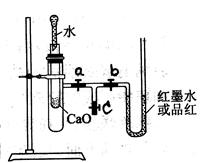
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com