
【题目】工业中很多重要的原料都是来源于石油化工,回答下列问题

(1)A的结构简式为__________________
(2)丙烯酸中含氧官能团的名称为__________________
(3)写出反应①的反应类型:_____________
(4)写出上述框图中某些过程的化学方程式:
过程②_________________________
过程④______________________
过程⑤_________________
(5)下列有关实验的说法中正确的是_______
A. 除去乙酸乙酯中的乙酸,可加入NaOH溶液,振荡后静置分液
B. 有机物C与丙烯酸属于同系物
C. 聚丙烯酸能够使酸性高锰酸钾溶液褪色
D. 除去硝基苯中混有的少量浓HNO3和H2SO4,可将其倒入到一定量的NaOH溶液中,振荡后静置分液
【答案】CH2=CH2 羧基 取代反应或硝化反应 CH2=CH2+H2O ![]() CH3CH2OH nCH2=CHCOOH
CH3CH2OH nCH2=CHCOOH![]()
 CH3COOH+CH3CH2OH
CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O D
CH3COOCH2CH3+H2O D
【解析】
B与丙烯酸发生酯化反应,生成丙烯酸乙酯,即B为CH3CH2OH,A与H2O反应生成乙醇,即A为CH2=CH2,B和C反应生成乙酸乙酯,即C为CH3COOH,然后进行逐步分析;
B与丙烯酸发生酯化反应,生成丙烯酸乙酯,即B为CH3CH2OH,A与H2O反应生成乙醇,即A为CH2=CH2,B和C反应生成乙酸乙酯,即C为CH3COOH,
(1)根据上述分析,A的结构简式为CH2=CH2;
(2)丙烯酸的结构简式为CH2=CHCOOH,含有官能团是碳碳双键和羧基,其中含氧官能团是羧基;
(3)苯转化成硝基苯,发生取代反应或硝化反应;
(4)过程②是乙烯与H2O发生加成反应,其反应方程式为CH2=CH2+H2O ![]() CH3CH2OH;过程④丙烯酸发生加聚反应,其反应方程式为nCH2=CHCOOH
CH3CH2OH;过程④丙烯酸发生加聚反应,其反应方程式为nCH2=CHCOOH![]()
 ;过程⑤发生的反应是CH3COOH+CH3CH2OH
;过程⑤发生的反应是CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O;
CH3COOCH2CH3+H2O;
(5)A、乙酸乙酯在NaOH溶液中能发生水解反应,因此除去乙酸乙酯中的乙酸不能用NaOH溶液,可以加入饱和碳酸钠溶液,振荡后静置分液,故A说法错误;
B、有机物C为CH3COOH,与丙烯酸含有的官能团不同,因此两者不互为同系物,故B说法错误;
C、根据过程④的反应方程式,聚丙烯酸中不含碳碳双键,不能使酸性高锰酸钾溶液褪色,故C说法错误;
D、HNO3和H2SO4与NaOH发生中和反应,生成NaNO3和Na2SO4,硝基苯不是溶于水的液体,然后采用分液的方法进行分离,故D说法正确。


 优翼小帮手同步口算系列答案
优翼小帮手同步口算系列答案科目:高中化学 来源: 题型:
【题目】有关如图装置的说法正确的是( )

A. 若K与c连接,石墨电极的电极反应为:O2+4e-+4H+=2H2O
B. 若K与c连接,则溶液中的Na+向铁电极移动
C. 若K与d连接,铁电极的电极反应为:2H2O +2e-=H2↑+2OH-
D. 若K与d连接,短时间后,加适量稀盐酸可使电解质溶液复原
查看答案和解析>>
科目:高中化学 来源: 题型:
【题目】二氧化氯(ClO2)是一种黄绿色气体,极易溶于水,在混合气体中的体积分数大于10%就可能发生爆炸,在工业上常用作水处理剂、漂白剂。回答下列问题:
(1)在处理废水时,ClO2可将废水中的CN-氧化成CO2和N2,该反应的离子方程式为_________。
(2)某小组通过NaClO3法制备ClO2,其实验装置如下图。
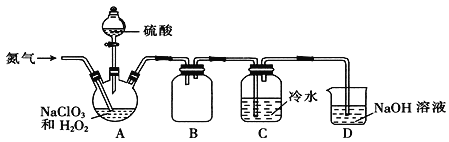
①通入氮气的主要作用有两个,一是可以起到搅拌作用,二是______________;
②装置B的作用是______________;
③装置A用于生成ClO2气体,该反应的化学方程式为______________;
④当看到装置C中导管液面上升时应进行的操作是______________。
(3)测定装置C中ClO2溶液的浓度:用______________(填仪器名称)取10.00 mLC中溶液于锥形瓶中,加入足量的KI溶液和H2SO4酸化,用0.1000 mol·L-1的Na2S2O3标准液滴定至溶液呈淡黄色,发生反应:I2+2S2O32-=2I-+S4O62-,再加入__________作指示剂,继续滴定,当溶液_______,即为终点。平行滴定3次,标准液的平均用量为20.00 mL,则C中ClO2溶液的浓度为________mol·L-1。
查看答案和解析>>
科目:高中化学 来源: 题型:
【题目】通过下列装置(部分夹持仪器已省略)可制取1,2-二溴乙烷。下列说法正确的是( )
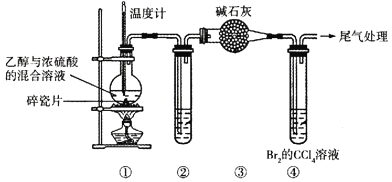
已知:烧瓶中反应后逸出的气体主要是CH2=CH2,含少量SO2、CO2及H2O(g)。
A.配制“乙醇与浓硫酸的混合溶液”时,将乙醇注入浓硫酸中并搅拌
B.②的试管中可盛放酸性KMnO4溶液以除去SO2
C.④中的Br2已完全与乙烯加成的现象是:溶液由橙色变为无色
D.可用分液漏斗从④反应后的混合物中分离出1,2-二溴乙烷并回收CCl4
查看答案和解析>>
科目:高中化学 来源: 题型:
【题目】资源化利用CO2,可以减少温室气体排放,还可以获得燃料或重要的化工产品。回答下列问题:
(1)CO2的捕集
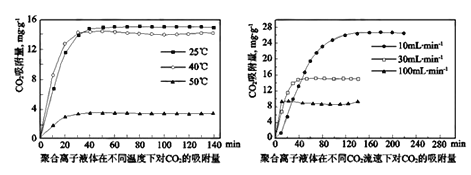
①用饱和Na2CO3溶液做吸收剂可“捕集”CO2。写出“捕集”CO2反应的离子方式_____________。
②聚合离子液体是目前广泛研究的CO2吸附剂。结合图像分析聚合离子液体吸附CO2的有利条件是_________________________。
(2)生产尿素:
工业上以CO2、NH3为原料生产尿素[CO(NH2)2],该反应分为二步进行:
第一步:2NH3(g)+CO2(g)H2NCOONH4(s) △H = - 159.5 kJ·mol-1
第二步:H2NCOONH4(s)CO(NH2)2(s)+ H2O(g) △H = +116.5 kJ·mol-1
①写出上述合成尿素的热化学方程式___________________________。该反应化学平衡常数K的表达式:_________________________。
②某实验小组模拟工业上合成尿素,在一定体积的密闭容器中投入4mol NH3和1mol CO2,实验测得反应中各组分物质的量随时间的变化如图所示:
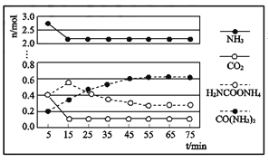
已知总反应的快慢由慢的一步反应决定,则合成尿素总反应的快慢由第__________步反应决定,总反应进行到___________min时到达平衡
(3)合成乙酸:中国科学家首次以CH3OH、CO2和H2为原料高效合成乙酸,其反应路径如图所示:
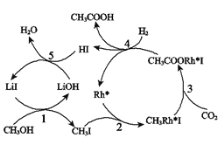
①原料中的CH3OH可通过电解法由CO2制取,用稀硫酸作电解质溶液,写出生成CH3OH的电极反应式_______________________。
②根据图示,写出总反应的化学方程___________。
查看答案和解析>>
科目:高中化学 来源: 题型:
【题目】(1)按系统命名法填写下列有机物的名称:![]() 名称是_____________________。
名称是_____________________。
(2)写出1,3-丁二烯与足量溴水反应的方程式:_______________________________。
(3)在有机物:①CH3CH3 ②CH2=CH2 ③CH3CH2C![]() CH ④CH3C
CH ④CH3C![]() CCH3 ⑤C2H6 ⑥CH3CH=CH2中,一定互为同系物的是________,一定互为同分异构体的是(填编号)________。
CCH3 ⑤C2H6 ⑥CH3CH=CH2中,一定互为同系物的是________,一定互为同分异构体的是(填编号)________。
(4)某有机物2.3克完全燃烧后,生成4.4克二氧化碳和2.7克水,测得该有机物的蒸气密度是2.054g/L(标准状况)。该有机物的分子式为______;请写出其可能的结构简式并命名____________________。
查看答案和解析>>
科目:高中化学 来源: 题型:
【题目】某人设计利用方案如下图所示:
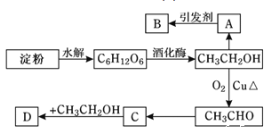
其中:A是水果催熟剂,B是高分子化合物,D是有水果香味的物质。
请回答以下问题:
(1)“C6H12O6”的结构简式是___________,C中原子团![]() 的名称是_______。
的名称是_______。
(2)写出下列转化的化学方程式及反应类型:
①A→B____________;反应类型______________。
②CH3CH2OH→CH3CHO_____________;反应类型____________。
查看答案和解析>>
科目:高中化学 来源: 题型:
【题目】DDT是人类合成得到的第一种有机农药,它的结构简式如图所示,有关它的说法正确的是( )

A. 它属于芳香烃B. 分子式为C14H8Cl5
C. 1mol该物质能与6mol H2加成D. 分子中最多可能有28个原子共面
查看答案和解析>>
科目:高中化学 来源: 题型:
【题目】化合物Y是一种常用药物,可由X制得。

下列有关化合物X、Y的说法正确的是
A.由X转化为Y发生取代反应
B.1 mol Y最多可与2 mol NaOH发生反应
C.用银氨溶液可检验X是否完全转化为Y
D.X与足量H2发生反应后,生成的分子中含有6个手性碳原子
查看答案和解析>>
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com